Pain Research Center Accelerates IRP Pain Studies
Dedicated Staff and Cutting-Edge Technology Helps Solve Pain’s Many Mysteries
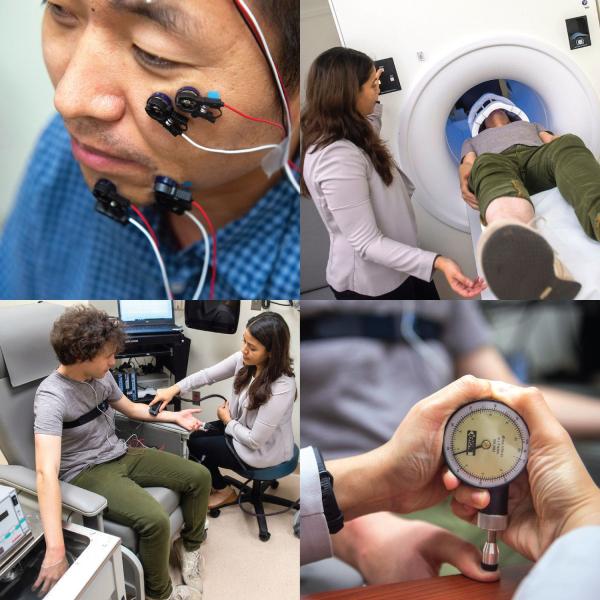
The NIH Pain Research Center provides IRP researchers access to a slew of resources to aid their efforts to learn more about pain.
For such a common ailment, pain remains a significant mystery. Part of the challenge of studying it is that it occurs in so many conditions and can vary from a mild ache to life-altering misery. Fortunately for both pain patients and IRP researchers studying pain, the NIH Pain Research Center has the technology and expertise to power new discoveries about pain in its many, complex forms.
On March 31 and April 1, NIH’s National Center for Complementary and Integrative Health (NCCIH) hosted a two-day virtual symposium titled “Tackling Pain at the National Institutes of Health: Updates From the Bench, the Clinic, and the New NIH Pain Research Center,” which featured presentations from a number of IRP scientists exploring important questions related to pain. Read on to learn more about some of the research discussed during that event, including efforts examining pain in patients with rare diseases, early-phase clinical trials of a new pain treatment, and investigations of how psychological factors can affect the way people experience pain. Then, check out last week’s blog post to read about the Center’s mission and the staff and cutting-edge tools that are helping accomplish it.
Studying Pain in Rare Diseases
Many common conditions can cause pain, from cancer and arthritis to everyday headaches and indigestion. However, pain is also a key component of many rare diseases. Several groups of IRP researchers are collaborating with the Pain Research Center to study the pain and other symptoms experienced by patients with rare diseases in the hopes that their research could yield benefits both for those particular patient populations and people with more common varieties of pain.
For example, one group of IRP researchers, led by senior investigator Paul Liu, M.D., Ph.D., is running a ‘natural history’ study to learn more about patients with mutations in the RUNX1 gene. People born with mutations in one copy of that gene suffer from a blood condition that leads to excessive bleeding and bruising and increases their risk of blood cancers like leukemia. As a long-time cancer researcher, Dr. Liu is particularly interested in how RUNX1 is involved in the development of leukemia, a cancer affecting the cells in bone marrow that produce blood cells. However, RUNX1 is also known to play a role in the development of certain pain-sensing neurons.
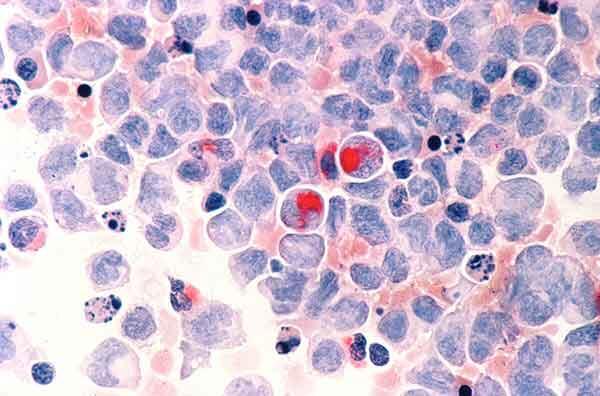
Human cells with acute myelocytic leukemia, one of the cancers for which patients with RUNX1 mutations are at increased risk.
Consequently, while monitoring the development of leukemia and its relationship to particular RUNX1 mutations is a major goal of the natural history study, the Pain Research Center has gotten involved to help assess other effects of RUNX1 mutations. This work involves both asking the participants about the symptoms they have experienced and running them through a battery of tests to measure different aspects of their pain and touch perception. Data collected so far seems to indicate that people with RUNX1 mutations might more frequently experience headaches and migraines compared to genetically typical individuals, and observations from Dr. Liu’s natural history study suggest that pain sensations may be altered in these patients.
“Some of our patients may have elevated levels or reduced tolerance of pain,” he says. “When we do bone marrow biopsies on participants, that can be quite painful. For many of our patients, we have to do sedation or more systematic anesthesia, rather than local anesthesia. The study from the Pain Research Center may help us understand why and figure out the best way to accommodate our patients, as well as tell us more about RUNX1’s contribution to pain in general.”
By partnering with the Pain Research Center, Dr. Liu and his colleagues will be able to create a more comprehensive picture of the syndrome caused by RUNX1 mutations. What’s more, because the gene is involved in a variety of biological processes, learning more about what happens when it does not work correctly could provide insights that extend beyond the patients participating in the study.
“RUNX1 is a master gene regulator,” Dr. Liu says, “so by learning about how it works, we may identify pathways related to RUNX1 that may be affecting other patients, even if they don’t have RUNX1 mutations.”
Investigating Pain That Outlasts Curative Therapies
Even in conditions that are so well-understood that they can actually be cured, the role of pain can still remain puzzling. One example of this is sickle cell disease, a genetic condition in which abnormally shaped red blood cells can trigger pain when they get stuck in small blood vessels. Bone marrow transplants and gene therapies can now cure patients with sickle cell by entirely correcting their blood cell abnormalities, yet some patients continue experiencing chronic pain even after receiving curative therapy.
To figure out a biological explanation for that persistent pain, the Pain Research Center is helping researchers in the Sickle Cell Branch at the National Heart, Lung, and Blood Institute (NHLBI) — led by IRP senior investigator Swee Lay Thein, M.B., B.S, D.Sc. — study the brains and pain responses of sickle cell patients after they receive a bone marrow transplant or gene therapy. Using a functional magnetic resonance imaging (fMRI) scanner, the researchers are examining how pain-related brain activity and the connectivity between various brain regions differs between sickle cell patients who have undergone one of those procedures and healthy individuals. Meanwhile, outside the scanner, the researchers are making similar comparisons about participants’ sensitivity to pain. One intriguing preliminary result from the study is that, even after curative therapy, sickle cell patients’ nervous systems may amplify pain signals more than those of healthy people, a phenomenon called central sensitization.

Dr. Eleni Frangos runs functional magnetic resonance imaging (fMRI) scans on volunteers to examine the brain activity of sickle cell patients after they have undergone curative therapies.
“I think the work that we’re doing is going to help not only patients who still have active sickle cell, but also post-curative therapy patients as well,” says the Pain Research Center’s Lead Scientific Officer, Eleni Frangos, Ph.D., who is working with Dr. Thein’s team on the study. “This is a unique situation in that we don’t often find a population where there’s a cure for the underlying condition that causes their chronic pain, but they seem to have ongoing pain after curative therapy for a variety of reasons. Eliminating the disease leaves us with the rest, which we can now probe to better understand the underlying mechanisms and apply that knowledge to other pain conditions.”
“The Pain Research Center has brought together an interdisciplinary group of people where the commonality is an interest in pain,” adds Dr. Thein. “The Pain Research Center is like a hub, and all the different diseases where pain is a commonality are the spokes, and we all can learn from each other.”
Creating Pain-Sensing Cells From Scratch
The best way to study something is usually to look at it directly, but that can be difficult when it comes to studying nerve cells. That’s why the labs of IRP senior investigators Alex Chesler, Ph.D., and Carsten G. Bönnemann, M.D., have teamed up to develop a way to take cells from an animal’s or person’s skin and turn them into sensory neurons. This enables researchers to directly examine the behavior of neurons with the same genetic makeup as the skin cells’ original donor.
“Being able to produce a virtually unlimited amount of sensory neurons in a petri dish gives us a lot of experimental access to this specific cell type, compared to the challenge of isolating sensory neurons from the complex tissue of an animal specimen,” explains Alec Nickolls, Ph.D., a postdoctoral fellow working on this research in Dr. Chesler’s lab. “It’s like the difference between getting your vegetables at the grocery store or foraging for them in the wilderness.”
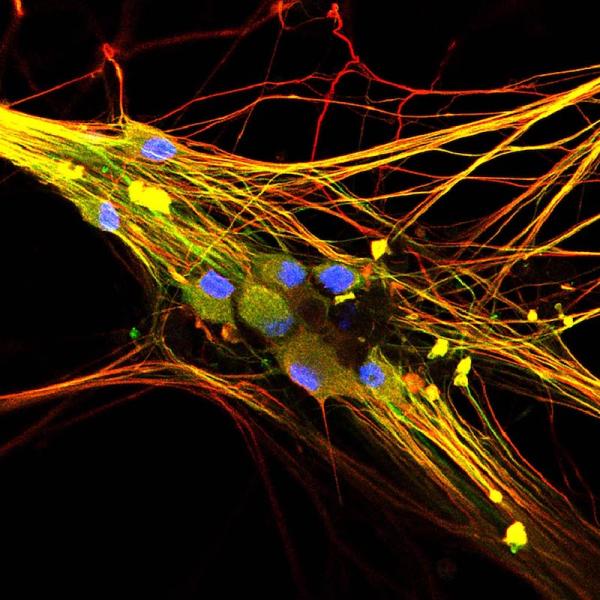
Human stem cell-derived sensory neurons
Already, Dr. Chesler’s team has used these lab-grown nerve cells to demonstrate the potential of a fat molecule called margaric acid for treating a condition called tactile allodynia, which causes normally benign touch like light brushing to feel painful. His lab is currently working on formulating a topical treatment containing margaric acid to test in animal studies and, ultimately, on human volunteers. Scientists could also use the lab’s technique to turn the skin cells of patients with chronic pain into sensory neurons in order to determine the specific problems that might be causing a patient’s pain and test personalized treatments that target those particular abnormalities.
“This technology can be used to derive sensory neurons from patients and ask how their personal genetics influence sensory neuron function,” says Dr. Chesler. “It can be used to figure out how variations in the genome contribute to diseases or chronic pain and to screen drugs on neurons from the patients to test specifically how the person might respond.”
These efforts will receive a considerable boost from the wide variety of data being collected by the Pain Research Center. By providing information about how people with certain illnesses or genetic variants perceive pain and other sensations, bench scientists like those in Dr. Chesler’s lab can more closely probe the relationship between those experiences and the way a person’s nerve cells work.
“The Pain Research Center is a valuable resource that allows us to work with a diversity of pain patients,” Dr. Nickolls says. “Going forward, we could potentially apply our technique to study patients with undiagnosed pain mechanisms that come through the Pain Research Center. In this way, the clinical features that the Center provides from each patient could be linked to genetic and physiological observations in the lab, giving us a clearer picture of how molecular-scale events give rise to certain types of chronic pain.”
Developing a new pain treatment
While many researchers at NIH search for compounds that can influence the pain-controlling systems and cells they have identified, some are already conducting clinical trials on particular drugs they believe to be promising. For example, a team of physician-scientists in the NIH Clinical Center’s Department of Perioperative Medicine, led by department chief Andrew Mannes, M.D., is focused on resiniferatoxin, a compound produced by several cactus-like plants native to Morocco and Nigeria.

Image credit: Valérie & Agnès, Wikimedia Commons
Euphorbia resinifera, more commonly called the resin spurge, is one of the cactus-like plant species that produce resiniferatoxin.
“The insight into how to use resiniferatoxin clinically to stop pain came directly from basic research into ion channels expressed in pain-sensing, or ‘nociceptive,’ neurons,” explains Dr. Michael J. Iadarola, Ph.D., a senior research scientist in the Department of Perioperative Medicine. “The particular channel that is activated by resiniferatoxin, TRPV1, is opened by physical heat and by the active ingredient in hot pepper, capsaicin. In fact, resiniferatoxin is a super potent analog of capsaicin and its high potency makes it particularly suitable for use as an interventional agent, and superior to capsaicin. The nerve endings that it binds to and activates are found throughout the body.”
Resiniferatoxin is not an opiate like oxycodone or morphine, so it is likely to be much less addictive than those two commonly used painkillers. Moreover, while opiate painkillers can lose effectiveness over time, resiniferatoxin works by selectively destroying the signal-transmitting ‘axons’ of pain-sensing neurons that utilize the TRPV1 ion channel. Depending on how the drug is given to patients, this effect can be permanent or can reverse over time. This makes resiniferatoxin a new approach to pain control for patients whose pain cannot be controlled through other means.
In one arm of this research, the Department of Perioperative Medicine is teaming up with the Pain Research Center and scientists in the Surgical Neurology Branch at the National Institute of Neurological Disorders and Stroke (NINDS), led by John Heiss, M.D., to evaluate resiniferatoxin as a treatment for cancer patients with chronic pain. The compound has previously shown promise in dogs with chronic pain due to cancer or osteoarthritis, a condition caused by the degradation of the protective tissue cushioning the ends of bones, as well as in a small phase 1 clinical trial with cancer patients conducted by Dr. Mannes’ team. That trial used lower doses of the molecule, so the group’s current study is measuring the effects of two different, higher doses. The study is still recruiting and collecting data, so specific results are not yet available, but Dr. Mannes describes them as “very encouraging.” His team is also conducting a clinical trial using resiniferatoxin to treat patients with osteoarthritis by injecting it directly into their knee joints.
In another project, led by IRP postdoctoral fellow Ellen Staedtler, M.D., Dr. Mannes’ team is testing resiniferatoxin as a treatment for Morton’s neuroma, an ailment that occurs when pressure on the foot damages a particular nerve, leading to chronic foot pain. Nearly a third of patients with Morton’s neuroma continue to experience moderate to severe pain after treatment with localized anesthetics, and even surgically removing the affected nerve does not alleviate pain in almost a fifth of patients.
Nurses in the Clinical Center’s Perioperative Medicine Department set up equipment for a surgical procedure.
Dr. Mannes’ group has designed a phase 1 clinical trial in patients with Morton’s neuroma to determine if using resiniferatoxin to destroy nerve endings in the affected foot is safe, and measures of pain relief will also be collected. Right now, though, the group is performing a variety of tests on patients with the condition to determine the best ways to evaluate how effectively resiniferatoxin alleviates their symptoms. This work utilizes ‘quantitative sensory testing’ (QST), which involves applying different types of painful and non-painful stimulation, such as a sharp poke or a source of heat, to the feet of patients and healthy individuals.
“This pilot testing is essential for understanding exactly how to test this area of the body and to establish a baseline in normal individuals and in patients before and after treatment,” says Dr. Iadarola. “It gives a quantitative objective endpoint for evaluation.”
“All the pilot studies for this research are being conducted at the Pain Research Center or with the aid of its personnel,” he adds. “It would have been very difficult to conduct these human studies without the Pain Research Center.”
What the researchers discover about the effects of resiniferatoxin on patients with Morton’s neuroma could have much broader applications as well.
“Morton’s neuroma results from physical damage to a peripheral nerve, and there are lots of other patients that develop chronic pain from nerve injury either following an accident or if a nerve gets nicked during a surgical procedure,” Dr. Iadarola says. “These people could be treated with resinifertoxin at the site of the nerve injury similar to the Morton’s patients. Also, people who develop phantom limb pain are potential candidates.”
Moving Beyond the Ouch
Pain may have evolved to make us aware that part of our body is injured, but that doesn’t mean only the affected body part is involved. Once pain signals from the body reach the brain, the brain can dramatically alter the way those signals are processed into a conscious perception of the pain experience. For this reason, it’s important to understand both the physical and psychological factors that influence pain.
The lab of IRP investigator Lauren Atlas, Ph.D., focuses primarily on the latter. Central to her work is the idea that how someone subjectively, personally experiences pain is different from the process of nociception — the body’s physical reaction to tissue damage.
“It is a person’s subjective experience that actually causes them to seek help,” she explains. “Suffering comes from the conscious experience of pain, and so to relieve pain, we want to understand what drives the subjective experience.”
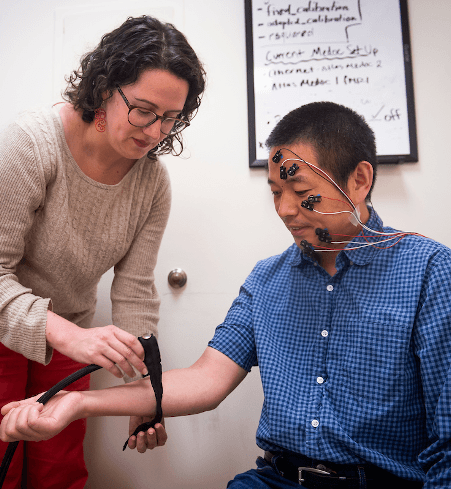
Dr. Atlas applies equipment to a volunteer to measure his nonverbal responses to painful stimulation.
During her time at NIH, Dr. Atlas’ team has made numerous discoveries about the way people experience pain. For example, she has shown that people’s individual sensitivities to pain are consistent across repeated tests, which is important information for determining how various interventions affect pain in an individual over time, as well as to figure out why pain sensitivity might differ between two healthy people or between people with and without a particular disease. Her work has also established that women’s experiences of pain are more consistent than those of males, a direct refutation of worries that led many past studies on pain to exclude women.
Nowadays, Dr. Atlas’ team is studying not only temporary, or ‘acute,’ pain but also the social context surrounding pain. Her group is particularly interested in how expectations shape pain and influence the effects of treatments via the placebo effect. One line of studies in her lab is examining whether stereotypical attitudes related to pain influence pain assessment, with the goal of improving our understanding of well-documented health disparities in pain and coming up with potential behavioral interventions. Her lab is also comparing pain with other emotional experiences to isolate aspects that are unique to pain.
Because Dr. Atlas’ research has mostly focused on studying acute pain in healthy volunteers, she has used the Pain Research Center to connect her with colleagues across the IRP who are studying pain in patient populations. Already, her group has begun collaborating with IRP senior clinician Janice Lee, D.D.S., M.D., an expert on disorders affecting bone development; IRP senior investigator Carlos Zarate, M.D., whose group is evaluating whether a potential new treatment for depression could also relieve pain; and researchers in the NIH Clinical Center’s Rehabilitation Medicine Department.
“We leverage the Pain Research Center to help us expand the scope of our research,” Dr. Atlas says. “The Pain Research Center will help us foster new collaborations with clinical groups focused on pain and to bring new tools to the study of pain. We look forward to support from the Center to help us facilitate these clinical collaborations.”
To learn more about the NIH Pain Research Center, check out our previous blog post discussing its goals, resources, and staff. And don’t forget to subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
Related Blog Posts
This page was last updated on Tuesday, May 23, 2023
