The Boon of Blood
A Look Inside NIH’s Department of Transfusion Medicine
A large team of scientists and technicians are needed to turn blood into life-saving treatments for patients at NIH.
The essential role of blood in our bodies has been recognized as far back as the time of ancient Greece, when the Greek physician Hippocrates included it in his list of four ‘humors’ that influence our health and emotions. Since then, scientists have vastly expanded our understanding of the dark red liquid running through our veins and arteries. Nowadays, researchers and technicians like those in NIH’s Department of Transfusion Medicine (DTM) can not only safely remove blood from one person and transfuse it into another, but they can also transform it into incredible forms of therapy.
In honor of World Blood Donor Day on June 14, the “I Am Intramural” blog took a look behind the scenes of the NIH Blood Bank to learn how a cadre of selfless donors supplies blood for patients at the NIH Clinical Center. However, other branches of DTM also play a vital role in treating patients enrolled in IRP clinical trials.
For example, before donated blood or blood-based therapies can be given to a patient, they must go through a series of preparatory steps performed in DTM’s Laboratory Services Section (LSS). In addition to processing, labeling, storing, and transporting blood, the LSS also matches donated cells with patients. People can only receive blood compatible with their blood type — for example, someone with O+ blood can only receive transfusions of O+ or O- blood — and certain patients must be given cells matched to them based on molecular tags called HLA markers.

DTM trainee Ashley Collins uses the NIH Clinical Center’s pneumatic tube system to send a blood component to another area of the hospital for transfusion into a patient.
Short for ‘human leukocyte antigen,’ HLA markers allow our bodies to distinguish between our own cells and foreign invaders like bacteria, viruses, and fungi. Unfortunately, when somebody receives a transfusion of blood or other cells that do not have the same HLA markers as their own, the recipient’s immune system may create antibodies that attack the foreign cells as if they were a dangerous infection rather than a medical treatment. This is not so much a problem for oxygen-carrying red blood cells, but it can be an issue when giving a patient transfusions of cells called platelets that help blood clot.
“Antibodies can cause transfusion reactions that can be quite severe, although luckily most of the time they’re not severe,” explains LSS Chief Bill Flegel, M.D. “The patient’s body recognizes that the transfused platelets don’t belong to him or her, and when we check the platelets, we see that the therapeutic effect we wanted is not achieved because the platelets are removed so quickly.”

During her treatment at the NIH Clinical Center, Sophia (center) received 66 units of donated platelets and 20 units of regular blood. As an added caution against transfusion-induced infections, the LSS ran most of the platelets Sophia received through a process called pathogen-reduction, which destroys any bacteria that might be hiding amongst the platelets.
People typically develop antibodies that thwart platelet transfusions only if they have undergone the procedure many times, but that scenario is fairly common for patients at the NIH Clinical Center due to their complex and chronic ailments.
“The patient cohort we have here at the NIH Clinical Center is typically highly treated, so they have had plenty of opportunities to produce antibodies and the percentage of patients with HLA antibodies is atypically high, so we have a high demand for HLA-matched platelets,” Dr. Flegel says.

Dr. Flegel (center) and two of his DTM colleagues receive a package of blood with a rare type sent all the way from Germany for a patient with severe aplastic anemia, a disease that disrupts the body’s production of blood cells.
HLA matching is even more important for the bone marrow or hematopoietic stem cell transplants used to treat blood cancers like leukemia and other illnesses that affect the blood. Once a donor is identified whose cells have the same or similar HLA markers to those of a patient at the NIH Clinical Center, either technicians in the NIH Blood Bank will collect blood-producing ‘hematopoietic’ stem cells from the donor’s blood or surgeons will collect some of his or her bone marrow in an operating room. This tissue is then processed by another branch of DTM, the Center for Cellular Engineering (CCE).
In addition to playing a crucial role in those life-saving transplants, the CCE also transforms blood cells into ‘cellular’ therapies. This includes gene therapies, in which a healthy version of a gene is inserted into cells from a person with a genetic illness, which are then grown in the lab before being re-infused back into the patient. The CCE also works with investigators at the National Eye Institute (NEI) to produce a cell-based treatment for the vision-destroying illness known as age-related macular degeneration. Much of the CCE’s efforts, however, go towards creating cell-based immunotherapies for cancer, such as CAR-T cells, immune cells that are genetically manipulated so they attack a patient’s specific cancer while leaving normal cells alone.
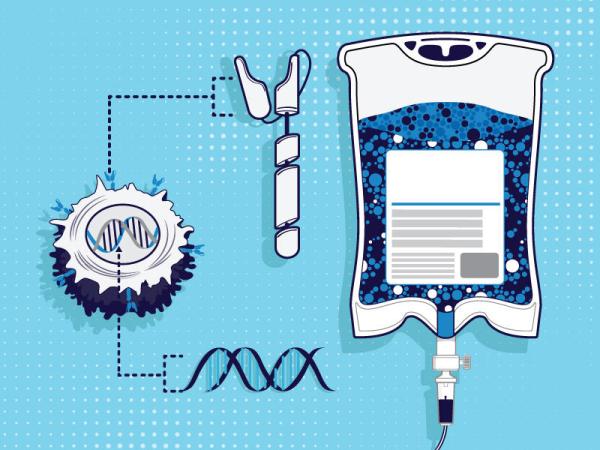
One of the CCE’s main tasks is turning immune cells isolated from the blood of cancer patients into personalized immunotherapies known as CAR-T cells.
“To treat cancer, there used to only be chemotherapy, surgery, and radiation therapy, but now we’ve added immunotherapy,” says CCE Director David Stroncek, M.D. “It’s important for clinicians at NIH to offer a full range of treatments so if they have a patient here, they can choose what options would be best and treat patients in the best way.”
The CCE also helps test new cellular therapies designed by scientists in other IRP labs. For those projects, the CCE relies on cells from individuals who are paid to donate blood for research purposes at the NIH Blood Bank, unlike the uncompensated volunteers who donate blood that is given directly to patients.
“When an investigator asks us to develop a new cellular therapy, we have to try it out on cells,” Dr. Stroncek explains. “A lot of places have to buy cells, but the Blood Bank has a program to collect cells for research from healthy subjects, and we use some of those to develop new cell processing methods for investigators.”
Last, but certainly not least, CCE researchers are constantly refining their procedures so they can make cellular therapies as efficiently as possible.
“A lot of times we set something up to make a cellular therapy, and then when we find out it’s effective and we’re going to produce more of it, we might go back and make a change to the manufacturing process or try a new manufacturing method,” Dr. Stroncek explains. “As time has gone on, new instruments and equipment have become available, so it’s important for us to see if they can be used in place of what we had before, and if we do use new equipment, to make sure the cells we produce are not different from those we made using the old way.”
In this video, CCE scientists and fellows describe the work they do to prepare blood components and cellular therapies for patients at the NIH Clinical Center.
Through their own work and their collaborations with researchers across the IRP, both Dr. Flegel and Dr. Stroncek see first-hand the incredible impact of the blood collected by the NIH Blood Bank. So much of what happens at NIH relies on selfless volunteers rolling up their sleeves to literally give a small part of themselves to those efforts.
“Blood donors really are having an important impact on biomedical research on the NIH campus,” Dr. Stroncek says.
“Some people aren’t aware how desperately that’s needed,” adds Dr. Flegel, who is a regular blood donor himself. “There are people who cannot donate for a variety of reasons, but the vast majority can and only a small fraction does. The important thing is to not only donate once in life — that’s not enough. You have to do it on a somewhat regular basis. You don’t have to max it out, but if someone were to donate one or two or three times a year, that would be of enormous help.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program, and check out last week's blog post to learn more about the NIH Blood Bank.
Related Blog Posts
This page was last updated on Wednesday, July 5, 2023
