Symposium Celebrates Award-Winning Female Scientists
Three Young Women Honored at Annual Event
There’s no doubt that science flourishes when it welcomes individuals with different backgrounds and perspectives. In pursuit of that goal, NIH has put considerable effort into closing the gender gap in the biomedical sciences, a field in which men significantly outnumber women, especially as the heads of labs and in leadership positions. Those efforts have so far yielded promising progress, with the proportion of women in IRP senior investigator positions increasing from 22 percent at the end of fiscal year 2016 to 27 percent at the end of fiscal year 2022. Over that same time period, women went from comprising 38 percent of IRP tenure-track investigators to 44 percent.
Part of what makes the IRP a welcoming place for female scientists is the NIH Women Scientists Advisors (WSA), a group of IRP scientists elected to represent the interests of women in the IRP. Once per year, the WSA selects a few female postdoctoral fellows or graduate students conducting research at NIH as WSA Scholars. At a symposium on April 13 honoring the achievements of this year’s Scholars, the awardees presented their efforts to learn more about a devastating childhood neurological condition, decrease health disparities in breast cancer, and use stem cells to investigate the roots of a nerve-destroying disorder. Read on to learn more about this year’s WSA Scholars and the important work they’re doing in their IRP labs.
Zoe Piccus: Scrutinizing Sphingolipids to Learn About a Neuron-Destroying Disease

Long before she enrolled as a Ph.D. student in the Brown University-NIH Graduate Partnerships Program (GPP), it was clear Zoe had the intense passion and curiosity that unites most scientists. The Massachusetts native was constantly chasing answers to whatever questions happened to come to mind.
“Both of my parents have an engineering background, so I grew up encouraged to ask how things work and how a well-functioning system can break down,” Zoe says.
The brain is a perfect example of a massively complex system capable of orchestrating a huge number of life-preserving bodily functions simultaneously. In 2018, as part of her graduate studies in the GPP, Zoe joined the lab of IRP investigator Claire Le Pichon, Ph.D., to investigate what happens when that carefully coordinated biological symphony falls apart.
Zoe’s research focuses in particular on the progressively paralyzing and eventually fatal neurological disease known as amyotrophic lateral sclerosis (ALS), which destroys the axons that nerve cells use to send electrical signals to one another. The disease is typically diagnosed in adulthood, but in 2021, a team of IRP researchers and collaborators outside NIH discovered that mutations in the SPLTC1 gene could cause a rare form of ALS that afflicts children. The mutations cause an over-production of fat molecules called sphingolipids, and Zoe is examining how those elevated supplies of sphingolipids cause symptoms of the disease.
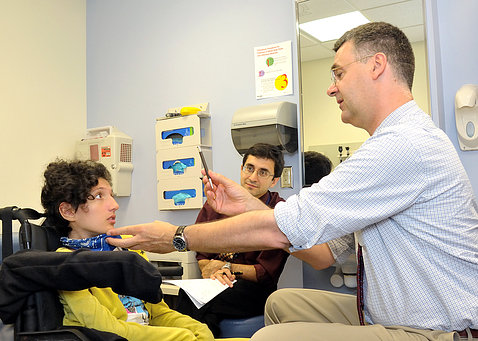
A team of NIH scientists led by IRP senior investigator Carsten G. Bönnemann (right) discovered in 2021 that mutations in the SPLTC1 gene caused ALS in a young girl from Italy (left).
“Sphingolipids are components of cell and organelle membranes throughout the body and are particularly abundant in the nervous system,” Zoe explains. “By recreating the ALS patient mutations in mice, we can monitor disease progression through motor behaviors and disease-related pathology.”
“This might help patients because we are developing a timeline for disease progression in these animals to serve as a basis for preclinical testing,” she continues. “These animals show axon degeneration resulting from the SPLTC1 mutation, so any potential therapy — perhaps to reduce sphingolipid levels — can be tested with this mouse line to see if it ameliorates degeneration and improves motor function. In the future, this can hopefully lead to new therapies for this rare childhood syndrome.”
Zoe has enjoyed witnessing the sheer breadth of projects going on around her in Dr. Le Pichon’s lab, where her colleagues study many different types of neuron-destroying ailments. Being constantly surrounded by that flurry of activity has helped Zoe establish connections that are benefiting her both scientifically and socially.
“My experience at the NIH has collaboration at the forefront,” Zoe says. “The Le Pichon lab environment is unlike anything I’ve experienced before. We are a group of researchers who respect and encourage each other’s scientific skills while having fun and not taking ourselves too seriously.”
Brittany Lord: Battling Breast Cancer Disparities

Growing up in Charleston, South Carolina, Brittany personally witnessed the tragic effects of cancer on her family and community, made all the more personal by the fact that African Americans like herself die from the disease at much higher rates than other groups. Listening to a genetic counselor who spoke to her high school science class one day, Brittany discovered an intense fascination for science that would eventually lead her to work that could reduce that racial health gap. She went on to earn a Ph.D. in genetics from the University of Georgia, where she first began investigating why different groups of people have such starkly different risks of developing cancer.
“I knew I had found my research calling once I started that work,” she says. “I was and am still very passionate about lowering the cancer mortality rate for Black men and women in this country.”
“Black women are 40 percent more likely to die from breast cancer than White women, a statistic that has remained stagnant for decades,” she adds. “I hope to contribute to a change in this narrative by identifying and understanding social and environmental risk factors that may differ between Black and White individuals in the U.S., as well as how those differences in risk factors can directly impact the biology of the tumor.”
As a postdoctoral fellow at NIH, Brittany is pursuing that goal under the mentorship of IRP senior investigators Stefan Ambs, Ph.D., M.P.H., and Gretchen Gierach, Ph.D., M.P.H., by examining how people’s neighborhoods affect a process called DNA methylation, which changes the behavior of genes. She specifically looks at a metric called neighborhood ‘deprivation,’ which combines a variety of measures that include the average income of a neighborhood’s residents and the proportion of its households that utilize public assistance and live below the poverty line. By linking research participants’ addresses to their neighborhoods, Brittany and her colleagues identified several cancer-related genes that are less methylated, and therefore function differently, in people from highly deprived neighborhoods. Sadly, but not surprisingly, her research has also found that Black people are much more likely to live in those neighborhoods than White people.

Brittany and her colleagues have found evidence that the neighborhoods people live in can affect their genes in ways that might influence their susceptibility to cancer and the course the disease takes.
“This work is so important, as our environments contribute to our health so much,” she says. “It is imperative that any work in cancer disparities includes both the social and biological determinants, as they both contribute to the worse cancer outcomes experienced by Black people in the U.S. Our work can not only inform how cancer develops and why it is more aggressive for certain populations, but can also identify specific biological differences that can be leveraged when creating cancer treatments that work for all populations.”
After spending more than three years as a Cancer Prevention Fellow at the National Cancer Institute (NCI), Brittany has learned a great deal from her mentors and colleagues, and she feels well-prepared to fulfill her goal of becoming an independent scientist after her fellowship ends. In addition to helping her career “blossom,” she says, working in the IRP has also allowed her to forge important relationships with her mentors and colleagues.
“I think my favorite part of this experience has been the connections I have been able to make with investigators at all levels, including the amazing trainees I get to work alongside every day,” she says. “I know the connections I have made here will carry on throughout my career.
Seungmi Ryu: Banking on Miniature Brains

Seungmi remembers loving her science classes as a child back in South Korea, and as she learned more, she found herself gravitating towards biology specifically. However, she has never let her special affinity for that field prevent her from venturing outside of it.
“In science, there’s physics, there’s chemistry, there’s biology,” she explains. “I’ve always liked biology because I feel like there’s story in it, compared to chemistry or physics where it’s something that you have to more just memorize equations. In biology, there’s a beginning and end to it, so I’ve been fascinated by biology, but I didn’t go into a biology department for my Ph.D. I wanted to be kind of an engineer — to make something out of biology and use it in different ways.”
There is no doubt that Seungmi has accomplished that vision at NIH, as she has spent the past four years working at the intersection of engineering and biology in the Stem Cell Translation Lab at the National Center for Advancing Translational Sciences (NCATS). In her lab, run by Acting Director Carlos Tristan, Ph.D., Seungmi creates ‘brain-in-a-dish’ models that she can use to learn about Friedreich ataxia, a rare genetic disease that destroys parts of the nervous system involved in balance and movement. These models, more formally known as ‘organoids,’ are created from induced pluripotent stem cells (iPSCs), which have the ability to transform — or ‘differentiate’ — into any type of cell found in the human body. For Seungmi’s studies, the iPSCs begin life as skin cells taken from either healthy donors or patients with Friedreich ataxia, which are then transformed into iPSCs. Next, Seungmi coaxes those iPSCs to form a three-dimensional structure that resembles a tiny version of the brain’s cerebellum, which plays a key role in coordinating movement and is the main part of the brain affected by Friedreich ataxia.
“Most of our current understanding of diseases is based on 2D, single-layer systems, or if they’re 3D, it’s from animal models like mice, or it’s from deceased donors if it’s human,” she says. “There’s a huge discrepancy between that and what’s going on in living human cells. I transform human cells into 3D organs that can recapitulate what’s happening in the human body.”
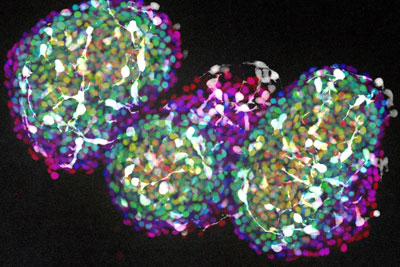
Three clusters of neurons grown from induced pluripotent stem cells (iPSCs). When grown in certain conditions, iPSCs can form into even more complicated, three-dimensional groups of cells that resemble miniature organs, including parts of the brain.
By comparing organoids generated from healthy people’s cells with those born from the cells of Friedreich ataxia patients, Seungmi and her labmates can glean important clues about what causes the disease.
“We want to find the molecular and cellular pathways that are different in them and then find therapeutics that can reverse that,” she explains.
Because her lab had only been around for a few years before she came to NIH, Seungmi played a key role in getting its research off the ground, and the state-of-the-art technology available in the IRP has made that effort all the more fruitful. She hopes to continue taking advantage of NIH’s unique resources to develop consistent methods of creating and studying organoids that scientists everywhere can rely on.
“It’s really nice how at NIH, we emphasize the reproducibility of the protocols that we develop,” she says. “We tested many different cell differentiation conditions in a high-throughput fashion to make sure that we are providing cues for stem cell differentiation at the right dose and at the right timepoint, whereas in academia you don’t have as much room to test all the possibilities because of the funding and because the instruments are different. I really appreciate how NIH emphasizes reproducibility in its science.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
Related Blog Posts
This page was last updated on Wednesday, July 5, 2023
