Alzheimer’s Patients Show Traces of Cellular Batteries in Blood
Biomarker Discovery Could Aid Diagnosis and Therapeutic Development
Traces of cells’ energy-producing mitochondria packaged inside tiny capsules called extracellular vesicles (EVs) floating in the blood may provide an easily measured biomarker for the diagnosis of Alzheimer’s disease, according to new IRP research.
Our cells can’t afford to be wasteful, so they prefer to recycle broken components. However, when the mitochondria that provide their energy are damaged beyond repair, cells may have no choice but to throw them out. New IRP research suggests that more of this mitochondrial debris floats in the blood of patients with Alzheimer’s disease, potentially providing an easy, cost-effective way to diagnose or even possibly predict the illness.1
The accumulation of toxic proteins in the brain causes a collection of severe memory problems and other cognitive issues known as ‘dementia’ in patients with Alzheimer’s disease. Because aging is the main risk factor for the condition and the average age of the American population is expected to increase over the coming decades, the number of people in the U.S. living with Alzheimer’s is predicted to triple from approximately 5.5 million today to nearly 14 million by 2060. In addition to the disease’s physical and emotional tolls, this increase could strain healthcare resources, since the only way to definitively diagnose Alzheimer’s is with invasive and expensive tests. Moreover, there is currently no effective treatment for the illness.
To address these issues, many researchers are now searching for biological signs of the disease, called biomarkers, that are cheap and simple to measure. Because drawing blood is easy and minimally invasive, IRP investigator Dimitrios Kapogiannis, M.D., has focused on identifying substances in the blood that might help clinicians diagnose Alzheimer’s disease, or even predict who might soon develop it.
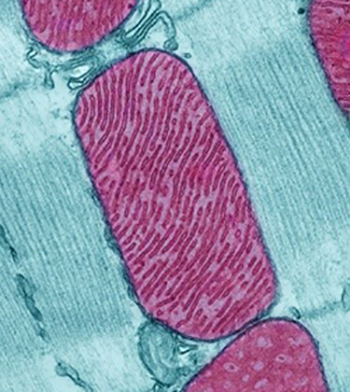
Image credit: Thomas Deerinck, National Center for Microscopy and Imaging Research
A mitochondria (purple) in a rodent heart cell.
“The brain is inaccessible for direct testing — we cannot take a brain sample,” says Dr. Kapogiannis, co-senior author of the new study. “Ideally, that would be the tissue we would need to examine to understand the disease, but because we cannot do that in living individuals, we have to rely on something that is accessible without invasive procedures, but may still give us relevant information.”
Dr. Kapogiannis’ research has specifically honed in on tiny capsules called extracellular vesicles (EVs) that cells release into the bloodstream to communicate with other cells and dispose of waste products. He had previously found that patients with Alzheimer’s have elevated levels of certain proteins in EVs ejected from neurons. In the new study, his lab teamed up with researchers led by IRP senior investigator Myriam Gorospe, Ph.D., the study’s other senior author, to investigate whether strands of genetic material called RNA might also be present in differing amounts in EVs from Alzheimer’s patients compared to healthy individuals.

More From the IRP
Research in Action
Changing Cells, Aging Bodies
While the researchers initially set out to measure all of the RNA molecules they could extract from EVs, they soon discovered that RNAs normally found only in cells’ energy-producing mitochondria “stood out head and shoulders over other RNAs,” according to Dr. Gorospe. The scientists took blood samples from individuals matched in age who were either healthy, had dementia due to Alzheimer’s disease, or had mild cognitive impairment (MCI) due to Alzheimer’s disease, a diagnosis signifying less severe cognitive deficits that are thought to occur as a result of the early stages of Alzheimer’s. Levels of numerous mitochondrial RNAs were significantly higher in EVs isolated from the blood of patients with Alzheimer’s dementia or MCI compared to those found in the blood of healthy individuals.
“People with MCI who have very mild symptoms and are very early in the course of the disease are already showing very high levels of these mitochondrial RNAs, so it’s a good marker for early detection,” says Dr. Gorospe. “You don’t have to have dementia for this to be a useful biomarker.”
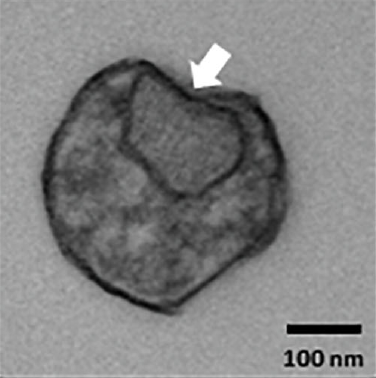
Transmission electron microscopy (TEM) image showing an extracellular vesicle containing structures (arrow) consistent with mitochondria fragments.
Of course, Alzheimer’s is a brain disease, so the results begged the question of whether the EVs the researchers analyzed came from the patients’ brains. To investigate this, Dr. Gorospe’s research team exposed isolated mouse neurons and various types of human brains cells growing in petri dishes to either harmful amyloid-beta proteins known to be involved in Alzheimer’s disease or hydrogen peroxide, a chemical that causes a damaging state known as ‘oxidative stress’ that is thought to afflict brain cells in Alzheimer’s patients. Compared to brain cells exposed to a harmless control solution, the cells exposed to amyloid-beta or hydrogen peroxide released EVs containing more mitochondrial RNA, just like those found in the blood of Alzheimer’s and MCI patients. What’s more, when examined under a powerful microscope, the scientists could actually see materials that closely resembled fragments of mitochondria in EVs released from brain cells exposed to the toxic agents. However, additional experiments will be needed to prove conclusively that what the IRP researchers saw were truly the remains of defunct mitochondria.
“It may look like a duck and quack like a duck, but now we have to show that it really is a duck,” says Dr. Kapogiannis.
If scientists can replicate the study’s results in much larger groups of volunteers, blood tests that measure levels of mitochondrial RNA in EVs could one day be used to identify people in the earliest stages of Alzheimer’s disease before symptoms become apparent. That could dramatically accelerate the development of Alzheimer’s therapies, since it would enable researchers to test potential treatments on patients very early in the course of the disease, when interventions are more likely to be effective.
“Biomarker development takes time and ultimately involves thousands of people and different cohorts from different populations and different racial backgrounds, and for that there is no shortcut,” Dr. Kapogiannis explains. “You don’t know how a biomarker operates in a large population until you examine it in a large population.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
References:
[1] Mitochondrial RNA in Alzheimer's Disease Circulating Extracellular Vesicles. Kim KM, Meng Q, Perez de Acha O, Mustapic M, Cheng A, Eren E, Kundu G, Piao Y, Munk R, Wood WH, De S, Noh JH, Delannoy M, Cheng L, Abdelmohsen K, Kapogiannis D, Gorospe M. Front Cell Dev Biol. 2020 Nov 16;8:581882. doi: 10.3389/fcell.2020.581882.
Related Blog Posts
This page was last updated on Wednesday, May 24, 2023
