Reining in Runaway Enzyme Halts Neuronal Destruction
Mouse Study Supports Potential Treatment Approach for Numerous Neurological Diseases
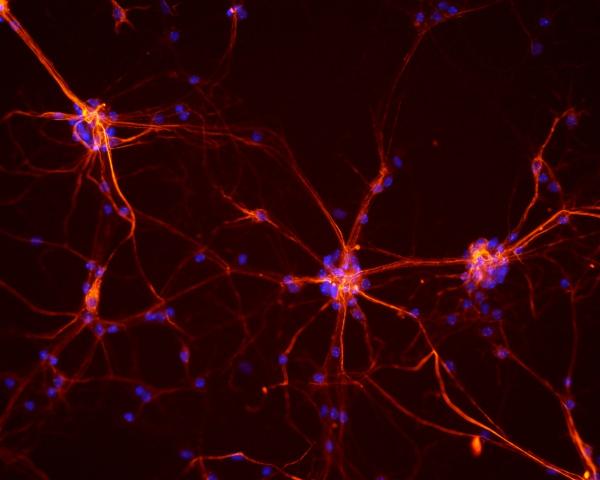
In mice with a particular ALS-causing mutation, a hyperactive enzyme called Cdk5 normally kills spinal cord neurons (pictured above). New IRP research has identified a promising way to reign in Cdk5 to prevent those toxic effects. Image courtesy of NICHD/S. Jeong.
Winter is fast approaching, bringing with it both picturesque snow flurries and raging blizzards. It's a good reminder that something that is desirable in moderate amounts can be downright dangerous in large quantities, and the systems that keep our cells healthy are no different. IRP researchers recently found a novel way to tamp down a runaway cellular process that can kill neurons, findings that may one day lead to new treatments for several debilitating neurological conditions.1
Just like our bones prevent our bodies from collapsing, cells also have skeletons — called cytoskeletons — that are vital to their survival. For cytoskeletal proteins to work correctly, they must have chemical tags called phosphate groups attached to them via a process called phosphorylation. A growing number of studies has found evidence that this process spins out of control in the brains of patients with diseases like Alzheimer’s disease and amyotrophic lateral sclerosis (ALS), a condition that causes muscle atrophy and progressive paralysis due to the death of the motor neurons that control movement.2,3
In neurons, an enzyme called Cdk5 phosphorylates two key cytoskeletal proteins, but only when it is attached to another protein called p35. A shorter form of p35, called p25, can also hook up with Cdk5, but when it does, it sends Cdk5 into overdrive, triggering a phosphorylation spree that can kill neurons.
“It’s very important that Cdk5’s activity is properly regulated,” says IRP senior investigator Harish Pant, Ph.D., the new study’s senior author. “Without Cdk5, the neuron cannot survive, but when Cdk5 becomes hyperactive, it causes disease.”
For years, Dr. Pant’s IRP team has worked on developing a shortened protein, or peptide, that could tame hyper-active Cdk5 but would not interfere with normal Cdk5 activity. In the group’s new study, they tested one of the results of that work — a compound called Cdk5 inhibitory peptide, or CIP — in mice with a genetic mutation linked to ALS in humans. The IRP scientists bred those mice with transgenic mice they had created whose motor neurons produce CIP, resulting in mice whose motor neurons carried the ALS-causing mutation and also manufactured CIP.
Both sets of mice with the ALS mutation had higher levels of the disease-causing p25 protein in their brains and spinal cords than mice without the mutation. However, Cdk5 was much more active in the mice with the mutation but no CIP than in the mice that had both the mutation and neurons that produced CIP. In addition, while many motor neurons died in the spinal cords of the former group of animals, leading to a shortened lifespan and significant movement difficulties later in life, the latter group did not experience any of these effects, suggesting that CIP was protecting neurons from the harmful effects of the ALS-causing mutation.
“CIP is selectively inhibiting the dysregulated Cdk5 activity, not the normal activity,” says Dr. Pant. “This is an encouraging discovery, and in the future perhaps clinicians can use this CIP molecule to treat humans.”
Motor neurons from the spines and brains of mice with the ALS mutation and no ability to produce CIP also had excessive phosphorylation of their cytoskeletal proteins, a phenomenon thought to be involved in diseases like Alzheimer’s and ALS. However, this runaway phosphorylation was markedly reduced in mice that could produce CIP, even when they had the ALS mutation.
CIP is particularly promising because it selectively affects only Cdk5 and no other similar enzymes, unlike other methods of taming over-active Cdk5 currently being pursued. However, the CIP molecule is too large to cross the blood-brain barrier, so Dr. Pant’s team is currently attempting to create a peptide compound with similarly selective and potent effects that is able to enter the brain via the bloodstream.
“This peptide technology is very new,” says Dr. Pant. “People are not very aware of using peptides for treating diseases like ALS and Alzheimer’s, but this approach will be very important to pursue further. Modification of these peptides will open doors for treating many types of diseases.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
References:
[1] Overexpression of the Cdk5 inhibitory peptide in motor neurons rescue of amyotrophic lateral sclerosis phenotype in a mouse model. Binukumar BK, Skuntz S, Prochazkova M, Kesavapany S, Amin ND, Shukla V, Grant P, Kulkarni AB, Pant HC. Hum Mol Genet. 2019 June 12. pii: ddz118. doi: 10.1093/hmg/ddz118.
[2] Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Patrick GN, Zukerberg L, Nikoli M, de la Monte S, Dikkes P, Tsai L.H. Nature. 1999 Dec 9; 402(6762):615-622.
[3] Cyclin-dependent kinase-5 (CDK5) and amyotrophic lateral sclerosis. Bajaj NP. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000 Dec;1(5):319-327.
Related Blog Posts
- Alzheimer’s Patients Show Traces of Cellular Batteries in Blood
- Dietary Supplement Powers Alzheimer’s-Afflicted Neurons
- Toxic Protein and Aging Combine Forces to Drive Brain Disease
- Mouse Study Supercharges Neurons to Detect Parkinson’s Disease
- Tiny Molecules Have Big Potential for Treating Eye Diseases
This page was last updated on Wednesday, May 24, 2023
