Tiny Molecules Have Big Potential for Treating Eye Diseases
Approach Could Protect or Even Regenerate Neurons in Eye and Spinal Cord

New IRP research suggests that tiny stands of genetic material called microRNAs could be useful treatment targets for the blinding eye disease known as glaucoma.
At the end of Aesop’s fable The Lion and the Mouse, the titular rodent saves his much larger friend from a hunter’s trap. Just like Aesop, scientists know well that even something tiny and often overlooked can lend a helping hand. Extremely short strands of genetic material called microRNAs, for instance, could make for useful therapeutic targets for glaucoma and other degenerative eye ailments, according to new IRP research.1
Everyone learns about DNA in middle school science class, and many people have at least heard of messenger RNA (mRNA) due to its role in two widely used vaccines for COVID-19. There is considerably less awareness, even among doctors and scientists, of much shorter strings of RNA called microRNAs. Like an annoying little brother, microRNAs pester their larger relatives and interfere with their ability to get anything done.
In the new study, researchers led by IRP senior investigator Stanislav Tomarev, Ph.D., examined the spectrum of microRNAs present in healthy and unhealthy retinal ganglion cells, which relay visual information to the brain via the optic nerve. The death of those cells causes glaucoma, the world's second-leading cause of blindness, and studying them can also yield insights into how scientists might prompt neurons in the spinal cord or brain to regenerate after injury.
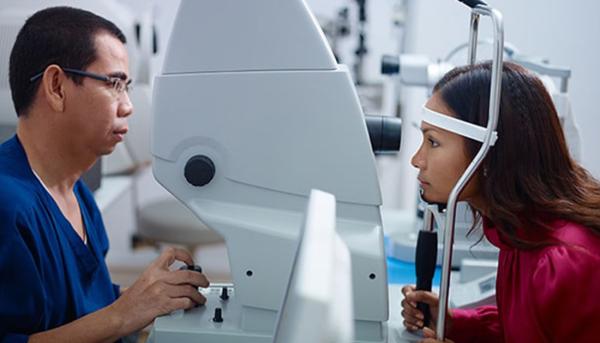
Eye doctors use the ‘air puff test’ — more formally known as non-contact tonometry — to measure the pressure inside the eye and determine a patient’s risk for developing glaucoma.
Right now, treatments for glaucoma focus on reducing pressure inside the eye, known as ‘intraocular pressure,’ since it is one of the main risk factors for glaucoma. However, therapies that target microRNAs could provide a useful additional treatment option. They could be especially helpful for patients who would otherwise require surgery to control intraocular pressure because medication failed to do so.
“In theory, if you can manipulate the microRNAs, you can prevent the retinal ganglion cells from dying even in the presence of elevated intraocular pressure,” Dr. Tomarev says.
Dr. Tomarev’s team compared the levels of various microRNAs in retinal ganglion cells from healthy rats, a rat model of glaucoma with elevated intraocular pressure, and a rat model of optic nerve trauma. The researchers identified more than 100 microRNAs whose abundance was significantly different in the healthy cells compared to the unhealthy cells. Moreover, increased intraocular pressure and optic nerve trauma had different effects on the levels of some microRNAs, suggesting that microRNA-based therapies for glaucoma might need to target different microRNAs than treatments for nerve damage stemming from an eye or spinal cord injury.
“Some signaling pathways may be more efficient for treating optic nerve trauma as opposed to glaucoma,” Dr. Tomarev says.

Glaucoma begins with the loss of peripheral vision, causing patients to percieve the world as if looking through a tunnel. Eventually, central vision degrades as well.
Next, with the help of the paper's first author, Ben Meade, Ph.D., a former postdoctoral fellow in Dr. Tomarev’s lab who is now an assistant professor at Cardiff University in the UK, Dr. Tomarev’s team manipulated the levels of certain microRNAs in retinal ganglion cells isolated from healthy rats and grown in the lab. The researchers specifically selected the five microRNAs whose levels had differed the most in the retinal ganglion cells of healthy and unhealthy rats in their prior experiment. Three of the selected microRNAs had been much more abundant in the unhealthy cells, so in the follow-up experiment the scientists used microRNA inhibitors to interfere with their ability to interact with other components of the cell. The other two selected microRNAs had been much less plentiful in the unhealthy cells, so in the follow-up experiment the scientists treated retinal ganglion cells with lab-created microRNAs designed to mimic those two microRNAs.
Retinal ganglion cells don’t typically survive for long when isolated and grown in the lab, but Dr. Tomarev’s team found that inhibiting two of the chosen microRNAs helped keep more of the cells alive for the three-day duration of the experiment, while targeting the other three microRNAs had no effect. What’s more, increasing the levels of two different microRNAs using microRNA mimics spurred the cells to grow more of the neuronal projections that they use to transmit electrical signals to the brain. Inhibiting two other microRNAs had the same effect, but to a lesser extent that did not quite reach statistical significance. Overall, the results suggest that manipulating microRNAs could be a viable approach for treating glaucoma or nerve injury. What’s more, according to Dr. Tomarev, it may have some advantages over other strategies.
Image credit: BruceBlaus via Wikimedia Commons
Manipulating the levels or activity of microRNAs in nerve cells could help treat not just glaucoma but also other forms of nerve damage like spinal cord injuries.
“MicroRNAs affect multiple mRNAs at the same time,” Dr. Tomarev explains. “Glaucoma is a very complex disease and there is no magic — no one single factor that can solve all problems. As a result, probably you can find a combination of individual microRNAs that will affect several signaling pathways at the same time, which could be very beneficial.”
Those cascading effects, however, mean that scientists like Dr. Tomarev will have to run many more experiments to determine a combination of microRNAs that preserves or regenerates retinal ganglion cells without too many side effects. Once the most promising microRNAs are identified, researchers will need to figure out the best way to deliver the treatments to cells and then test them in larger animal models whose eyes more closely resemble our own.
“Animal models don’t mimic a hundred percent what is going on in the human eye,” Dr. Tomarev says. “In this sense, to prove that a treatment works in larger animal models that mimic the human eye much better is a very important step. The goal is not to protect retinal ganglion cells in mice or rats, but to apply this information in humans.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
References:
[1] miRNA Changes in Retinal Ganglion Cells after Optic Nerve Crush and Glaucomatous Damage. Mead B, Kerr A, Nakaya N, Tomarev S. Cells. 2021 June 22;10(7):1564. doi: 10.3390/cells10071564.
Related Blog Posts
- Alzheimer’s Patients Show Traces of Cellular Batteries in Blood
- RNA-Targeting Therapeutic Restores Protein Absent in Spinal Muscular Atrophy
- Global Scientists Come Together at the National Institutes of Health
- Dietary Supplement Powers Alzheimer’s-Afflicted Neurons
- Reining in Runaway Enzyme Halts Neuronal Destruction
This page was last updated on Tuesday, January 30, 2024
