Mouse Study Supercharges Neurons to Detect Parkinson’s Disease
Pushing Cells to Their Limits Could Enable Earlier Diagnosis and Treatment

Supercharging certain neurons in the brain to test their limits allowed IRP researchers to detect Parkinson’s disease in mice in its very early stages.
If the many stories of mothers lifting cars to save their trapped children prove anything, it’s that we cannot know the true capabilities of our bodies until they are put to the test. This concept, it turns out, could be the key to much earlier diagnosis for Parkinson’s disease. By stimulating specific neurons to push them to their limits, IRP researchers were able to detect Parkinson’s in mice in its very early stages, opening up the possibility that a similar test could one day allow human patients to begin treatment before the disease has caused too much damage.1
Parkinson’s disease is caused by the death of neurons in the brain that release a chemical called dopamine, and the loss of those cells impairs patients’ ability to move. However, those tell-tale symptoms don’t show up until people have lost more than half of the dopamine neurons in their brains, a process that can begin years or even decades before they receive a diagnosis.
“Parkinson’s is a progressive disease, so the earlier we can diagnose it, the earlier we can start the intervention, which can help to slow the progression of the disease,” says IRP investigator Guohong Cui, M.D., Ph.D., the new study’s senior author.

Photo credit: Steve McCaw
Dr. Guohong Cui, the new study’s senior author.
Unfortunately, early diagnosis for Parkinson’s disease has long been a challenge. Many researchers are hoping to use a malformed protein called alpha-synuclein as a ‘biomarker’ to detect Parkinson’s disease, but so far it’s unclear whether differences in that protein between Parkinson’s patients and healthy people can be detected early in the course of the disease.
Dr. Cui’s lab, on the other hand, is pursuing an entirely different strategy. His approach measures dopamine ‘metabolites,’ which are molecules produced when dopamine that has been released from neurons is broken down. Unlike dopamine itself, some of these metabolites can pass through the blood-brain barrier into the bloodstream. They also show up in the cerebrospinal fluid (CSF) surrounding the brain and spinal cord.
“If you look at the average, Parkinson’s patients show lower levels of dopamine metabolites in CSF samples,” Dr. Cui explains. “The issue is that the difference is very small because the variation among patients is large, so the sensitivity of this measure will not be good enough as a diagnostic method. Our idea is to increase the dynamic range so we can make these two groups — the patients and the healthy control group — more different from each other.”
To test this idea, Dr. Cui’s team stimulated the dopamine neurons of 20-week-old mouse models of Parkinson’s disease by giving them two FDA-approved drugs: haloperidol, which induces dopamine neurons to release more dopamine, and methylphenidate, which blocks cells from recapturing the dopamine they’ve released. At that age, the mice had only lost 28 percent of their dopamine neurons, too few to cause noticeable symptoms. However, Dr. Cui hoped that giving the drugs to the Parkinson’s mice and a group of healthy control mice would lead to detectable differences in two dopamine metabolites, DOPAC and HVA, in the blood and CSF of the two sets of animals.
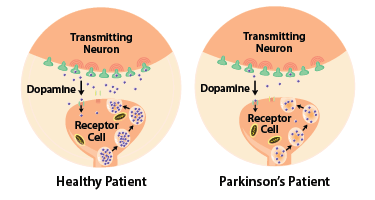
Dr. Cui’s test takes advantage of the fact that Parkinson’s disease diminishes the brain’s ability to produce dopamine.
That is exactly what his team found. While levels of the two metabolites in the CSF and blood were initially no different in the healthy mice and Parkinson’s mice, one hour after both groups were given the two drugs, the CSF of the Parkinson’s mice contained significantly less HVA and DOPAC than CSF from the healthy animals, and their blood had significantly less HVA as well. These differences in dopamine metabolites could even be detected when the same experiment was repeated with 15-week-old mice, a time point so early in the course of the illness that the mouse models of Parkinson’s disease and their healthy counterparts still had the same number of dopamine neurons in their brains.
In this way, using drugs to test the limits of the brain’s ability to produce dopamine revealed that the brains of the Parkinson’s mice couldn’t keep up with those of their healthy counterparts, even in the very early stages of the disease. If these results are replicated in human patients, a diagnostic test based on this approach could one day identify Parkinson’s disease in people before the illness has progressed far enough to affect movement.
“The most exciting moment for me was when I had this idea,” Dr. Cui recalls. “I was very eager to test it. I thought this was a very clever, interesting idea, and a very testable idea, so we just went ahead and tested it, and the result was consistent with our predictions.”
If Dr. Cui’s results can be reproduced in people with Parkinson’s, it may one day be possible to use a simple blood test to identify individuals in the illness’ early stages. The blood test’s results would likely be confirmed with a test of the patient’s cerebrospinal fluid, which can be collected using a routine procedure.
Despite the study’s promising results, haloperidol’s side effects may be an obstacle to using it for diagnosing early-stage Parkinson’s in humans, although Dr. Cui’s study did show that methylphenidate can partially alleviate haloperidol’s side effects in mice. Consequently, Dr. Cui’s lab is testing two experimental drugs to see if they can replace haloperidol in the diagnostic test while causing fewer side effects. At the same time, his lab is working on treatments that can slow down the loss of dopamine neurons in Parkinson’s patients, since diagnosing the illness in its early stages would not be nearly as helpful without such therapies.
“Right now, we don’t have any really early markers of Parkinson’s disease that we can detect, so we can only begin treating patients after the clinical onset,” Dr. Cui says. “We have missed the best time window when we can really slow the progression. Once we have a method to do early diagnosis, we’ll be able to apply those interventions early on to change the course of the disease. That will be a game-changer.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
References:
[1] Zhou J, Li J, Papaneri AB, Kobzar NP, Cui G. Dopamine Neuron Challenge Test for early detection of Parkinson's disease. NPJ Parkinsons Dis. 2021 Dec 16;7(1):116. doi: 10.1038/s41531-021-00261-z.
Related Blog Posts
This page was last updated on Wednesday, May 24, 2023
