IRP’s Richard Youle Receives Breakthrough Prize
‘Silicon Valley Nobel’ Recognizes Groundbreaking Parkinson’s Disease Research

NIH senior investigator Richard Youle received the 2021 Breakthrough Prize in Life Sciences for his discoveries about the biological roots of Parkinson’s disease. Photo by Matt Elliott, courtesy of the MRC Protein Phosphorylation and Ubiquitination Unit, University of Dundee
It can be easy to underestimate the value of so-called ‘basic science’ that doesn’t appear, upon first glance, to have clear therapeutic applications. One of the hidden strengths of this sort of work is its ability to link seemingly disparate areas of scientific inquiry by identifying commonalities between the structure or behavior of different biological molecules. By following these unexpected connections over the course of his career, IRP senior investigator Richard Youle, Ph.D., has made critical discoveries about Parkinson’s disease — research that this year earned him the prestigious Breakthrough Prize in Life Sciences.
Founded in 2013 by a group that includes Google co-founder Sergey Brin, Facebook CEO Mark Zuckerberg, and 23andMe CEO Anne Wojcicki, the Breakthrough Prize in Life Sciences acknowledges “transformative advances toward understanding living systems and extending human life.” Commonly considered to be Silicon Valley’s answer to the Nobel Prize, the award comes with a $3 million windfall. One prize each year is reserved for work related to Parkinson’s disease and other disorders that result from the death of neurons in the brain.
“It’s such an honor, and it also honors my graduate students and postdocs who worked on this with me for a decade,” says Dr. Youle, head of the Biochemistry Section at the NIH’s National Institute of Neurological Disorders and Stroke (NINDS). “I think it reflects well on the [NIH] intramural program and the freedom NIH has granted me to follow different meandering research paths.”
At the outset of his career, Dr. Youle could scarcely have imagined he would make key contributions to the study of Parkinson’s disease, a neurological disorder that results from the destruction of ‘dopaminergic’ neurons that control movement using the chemical dopamine. His graduate research focused on finding the cellular location of the highly toxic chemical ricin in the seeds of the castor oil plant. Dr. Youle’s expertise with ricin led to a postdoctoral position at the NIH in the lab of former IRP investigator David Neville, M.D., who wanted to develop cancer treatments by linking toxic chemicals to molecules called monoclonal antibodies that attach selectively to tumor cells. Under Dr. Neville’s tutelage, Dr. Youle learned about diphtheria toxin, which shares a name with the infectious disease caused by the bacteria that produce it.
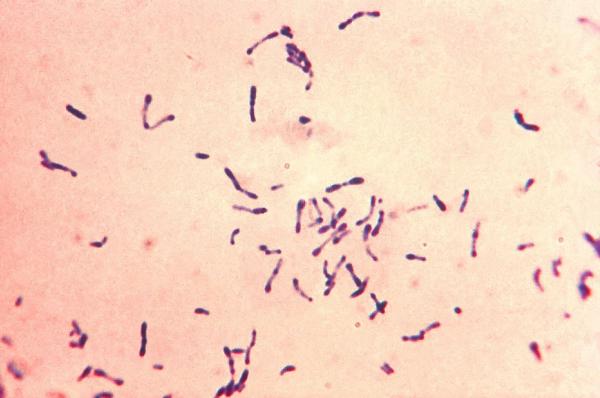
When Dr. Youle first came to the NIH, his research focused on the diphtheria toxin produced by the bacterium Corynebacterium diphtheriae, pictured here.
When Dr. Youle started his own lab at NINDS, he applied his expertise on ricin and diphtheria specifically to combating brain cancer. The direction of his work took a turn, however, when medical device company Abbott Laboratories reported striking structural commonalities between parts of the diphtheria molecule and another molecule belonging to the Bcl-2 family of proteins, which were known to be involved in a cellular self-destruct system called apoptosis. Dr. Youle’s lab subsequently found that another member of the Bcl-2 family, called Bax, moves from other parts of the cell and wedges itself into the outer surface of cells’ energy-producing mitochondria, where it plays a key role in triggering cell death.1
“We were just following the molecules to mitochondria,” Dr. Youle explained. “I had never studied mitochondria before, but because Bax translocates to mitochondria, we started studying mitochondria.”
This new focus on mitochondria led a medical student in Dr. Youle’s lab to investigate a protein called parkin, produced by a gene that leads to early-onset Parkinson’s disease when it does not work properly. Other researchers had discovered that flies with parkin mutations had malfunctioning mitochondria, and Dr. Youle’s team discovered that parkin attaches to the outer surface of damaged mitochondria, just like Bax does when a cell initiates apoptosis. What’s more, the group found that cells exposed to mitochondria-attacking toxins soon lost their mitochondria.
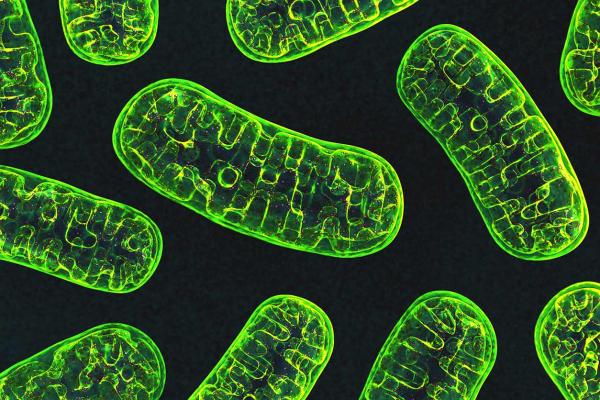
Mitochondria, pictured here, are the ‘powerhouses’ of the cell.
“I remember vividly the day he showed me his results,” Dr. Youle says. “There was no precedent for this in the scientific literature. To see this damage-induced removal of mitochondria was stunning. We were in territory where nobody had ever been before.”
Over the next decade, studies from Dr. Youle’s lab created a detailed picture of why mutations in parkin and another gene, called PINK1, lead to early-onset Parkinson’s disease. His team discovered that the PINK1 molecule accumulates on unhealthy mitochondria, where it modifies chains of a molecule called ubiquitin. Parkin binds to these modified strands of ubiquitin, which triggers it to add more ubiquitin chains to the outer surface of mitochondria for PINK1 to modify.2 In healthy cells, this positive feedback loop eventually attracts cellular machinery that remove the dysfunctional mitochondria, but in cells with parkin or PINK1 mutations, this quality control system fails and the defective mitochondria stick around. For reasons that are still not well-understood, the inability to get rid of malfunctioning mitochondria causes dopaminergic neurons to die, leading to the movement difficulties associated with Parkinson’s disease.3
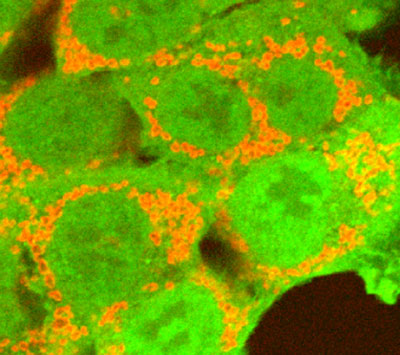
The parkin protein (green) marks damaged mitochondria (red) so cells know to get rid of them.
“Our research really linked these two genes like hand and glove because the product of PINK1 activates parkin and the product of parkin is a target of PINK1,” Dr. Youle explains. “It’s really an elegant molecular understanding of how these two Parkinson’s disease genes work together.”
The discoveries made in Dr. Youle’s lab have triggered a flurry of efforts by pharmaceutical companies to develop methods of treating Parkinson’s disease by revving up the process of mitochondrial disposal controlled by parkin and PINK1. Meanwhile, Dr. Youle’s work remains anchored in basic science, as his lab attempts to determine how problems with mitochondrial quality control kill dopaminergic neurons. Just as it has throughout his career, the NIH Intramural Research Program continues to provide Dr. Youle and his team the freedom to shift focus and pursue whatever curiosities might pop up in the course of their ground-breaking work.
“I’ve heard the saying that successful science impedes further successful science because you develop a dogma and you tend to defend that dogma in the later period of your career,” Dr. Youle says. “For me, mid-way through my career, I can enter a new field. I’m like a young postdoc. You have to really study it and you’re stressed, but you also bring a fresh perspective and you’re youthful again.”
References:
[1] Bax translocation to mitochondria subsequent to a rapid loss of mitochondrial membrane potential. Smaili SS, Hsu Y, Sanders KM, Russell JT, Youle RJ. Cell Death Differ. 2001 Sep;8(9):909-20. doi: 10.1038/sj.cdd.4400889.
[2] PINK1 phosphorylates ubiquitin to activate Parkin E3 ubiquitin ligase activity. Kane LA, Lazarou M, Fogel AI, Li Y, Yamano K, Sarraf SA, Banerjee S, Youle RJ. J Cell Biol. 2014 Apr 28;205(2):143-53. doi: 10.1083/jcb.201402104.
[3] Endogenous Parkin Preserves Dopaminergic Substantia Nigral Neurons following Mitochondrial DNA Mutagenic Stress. Pickrell AM, Huang C, Kennedy SR, Ordureau A, Sideris DP, Hoekstra JG, Harper JW, Youle RJ. Neuron. 2015 Jul 15;87(2):371-81. doi: 10.1016/j.neuron.2015.06.034.
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
Related Blog Posts
- Antiviral Drug Stems the Spread of Parkinson’s-Promoting Protein
- IRP Scientists Win Breakthrough Prize for Parkinson’s Discoveries
- IRP’s Peter Basser Elected to the National Academy of Engineering
- Neuron-Killing Protein Exploits a Vulnerability in Mitochondrial Armor
- Three-Minute Talks Showcase Smooth-Talking Scientists
This page was last updated on Wednesday, July 5, 2023
