Antiviral Drug Stems the Spread of Parkinson’s-Promoting Protein
Study Shows Promise of New Treatment Approach in 3D Brain ‘Organoid’ Model

Recent IRP research points to a promising new approach for slowing or stopping the progression of movement problems that affect people with Parkinson’s disease.
Our cells’ survival depends on their ability to take in the nutrients and other substances that they require. Unfortunately, this capacity is a double-edged sword, as cells can bring in both valuable resources and ticking time bombs. However, IRP researchers recently identified an existing drug that may be able to combat Parkinson’s disease by reducing cells’ penchant for snatching up the toxic proteins involved in Parkinson’s disease.1
Parkinson’s disease stems from the death of ‘dopaminergic’ neurons in the brain, which use a chemical called dopamine to communicate with other cells. Without those neurons, the body has trouble initiating and controlling movements. Current treatments aim to restore levels of dopamine in the brain, but doing so is far from a panacea.
“By providing dopamine, we can alleviate symptoms, but that doesn’t prevent the death of the cells that produce dopamine,” explains IRP senior investigator Yihong Ye, Ph.D., the new study’s co-senior author.
Dr. Ye’s lab has been trying to improve Parkinson’s treatment by investigating the mechanisms through which toxic proteins kill neurons. This includes a cellular process called ‘endocytosis’ that allows cells to bring in substances like nutrients from their surroundings.
“It’s usually used by cells for good purposes,” Dr. Ye says, “but quite often cells cannot distinguish good things from bad things, so they can also take in garbage — in this case, a misshapen form of alpha-synuclein.”
Alpha-synuclein is a protein that usually exists in single units, but when it is abnormal, those units meld together into long strands that scientists called ‘aggregates’ or ‘fibrils,’ which are toxic to neurons.
“Alpha-synuclein fibrils can spread from one part of the brain to another, and as the disease worsens, you see more and more aggregates accumulating in patients’ brains,” Dr. Ye says. “This type of spreading behavior requires cells to take in the protein and then release it into another part of the brain. My lab has spent a lot of time thinking about ways to block this cell-to-cell spreading of the abnormal alpha-synuclein protein.”

IRP researchers use robots like these to run drug ‘screens’ that can quickly identify promising treatment candidates among thousands of existing drugs.
That line of inquiry led to a collaboration with another IRP researcher, Wei Zheng, M.D., Ph.D., whose team helped Dr. Ye’s group run a drug ‘screen’ to quickly assess the effects of many different medications on cells’ intake of alpha-synuclein fibrils.2 The study flagged a drug called Tilorone, which is not approved for sale in the U.S. but is used to treat several viral illnesses in other parts of the world. However, that screen and subsequent studies used flat, two-dimensional collections of cells to gauge Tilorone’s effects — a useful starting point, but far from a human brain. Consequently, in their new study, Dr. Zheng’s and Dr. Ye’s research teams worked together to test Tilorone’s effects in a three-dimensional ‘midbrain organoid’ model.
“The 2D model we used before consisted of just pure neurons, so it doesn’t have the other cell types that we see in the human brain, whereas the 3D model has all those cell types plus a structure and organization that is more similar to the human brain,” Dr. Ye says.
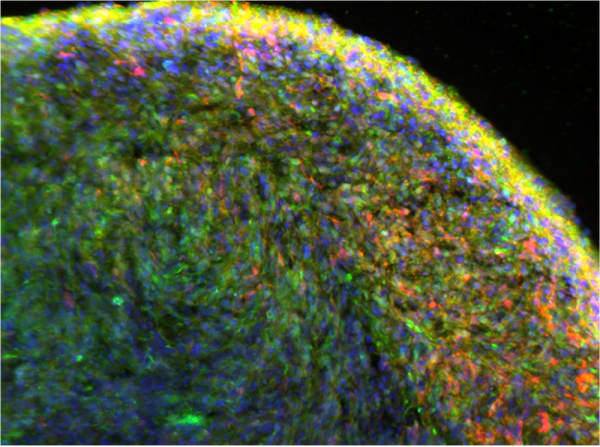
A close-up view of the organoids developed by Dr. Zheng’s team, showing neurons in red and alpha-synuclein in green.
“The 3D model has advantages over 2D models because it mimics more the three-dimensional human brain and also includes interactions between different cell types,” Dr. Zheng adds.
As expected, exposing the organoids to alpha-synuclein fibrils caused the neurons in them to die. However, Tilorone diminished this cell death by not only reducing the amount of alpha-synuclein that neurons in the organoids’ outermost layers took in, but also by inhibiting the spread of the toxic protein from the organoids’ surface to cells in its inner layers.
“Imagine alpha-synuclein fibrils are like a virus,” says the study's first author, Qi Zhang, Ph.D., a research fellow in Dr. Zheng’s lab. “They are transmitted from a sick cell to the nearby healthy cells in the brain, but we found that Tilorone can reduce that. This is quite remarkable because it means we can potentially slow down or stop the disease’s progression.”
The study also found that while exposure to alpha-synuclein fibrils increased the amount of ‘hyper-phosphorylated’ alpha-synuclein in the organoids — the kind particularly associated with Parkinson’s disease — Tilorone treatment blunted that increase. The medication similarly reduced elevation of inflammatory proteins in organoid cells caused by alpha-synuclein fibrils, as well as the toxic protein’s effects on other proteins involved in the functioning of cells’ energy-producing mitochondria.
“There are many genes involved in mitochondria that have been linked to Parkinson’s disease,” Dr. Ye says. “Dopaminergic neurons are one of the types of neurons in the brain that are extremely dependent on energy consumption. They consume energy like crazy, so when mitochondria are not functional, those cells are the most vulnerable ones.”

Study lead author Qi Zhang (left) and co-senior authors Wei Zheng (middle) and Yihong Ye (right).
While research into Tilorone as a treatment for Parkinson’s disease is only in its early stages, the results of the new study were encouraging enough to get Dr. Zheng’s and Dr. Ye’s teams excited about partnering with other researchers to begin studies of the medication’s effects on animal models of Parkinson’s disease, with a particular eye toward observing its short- and long-term side effects. If the side effects are not too severe, or the drug can be modified to reduce them while maintaining its effectiveness, it could one day provide a new way to slow the progression of Parkinson’s disease with a simple daily or weekly pill.
“This medication is pretty amazing,” Dr. Zhang says. “It doesn’t just reduce the uptake of these toxic alpha-synuclein fibrils, but it also can modulate some pathways that are involved in neuronal survival. It has good potential to be further pushed into clinical studies.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
References:
[1] Zhang Q, Liu M, Xu Y, Lee J, Jones B, Li B, Huang W, Ye Y, Zheng W. Tilorone mitigates the propagation of α-synucleinopathy in a midbrain-like organoid model. J Transl Med. 2024 Sep 2;22(1):816. doi: 10.1186/s12967-024-05551-7.
[2] Zhang Q, Chen CZ, Swaroop M, Xu M, Wang L, Lee J, Wang AQ, Pradhan M, Hagen N, Chen L, Shen M, Luo Z, Xu X, Xu Y, Huang W, Zheng W, Ye Y. Heparan sulfate assists SARS-CoV-2 in cell entry and can be targeted by approved drugs in vitro. Cell Discov. 2020 Nov 4;6(1):80. doi: 10.1038/s41421-020-00222-5.
Related Blog Posts
This page was last updated on Tuesday, December 10, 2024
