IRP Scientists Win Breakthrough Prize for Parkinson’s Discoveries
Andrew Singleton and Ellen Sidransky Lauded for Genetics Research

Dr. Ellen Sidransky (left) and Dr. Andrew Singleton (right) received the 2024 Breakthrough Prize for discovering two of the most common genetic contributors to Parkinson’s disease.
Throughout history, some of the most important insights about devastating illnesses have come from identifying genes that contribute to them. Parkinson’s disease, a neurological condition that robs patients of the ability to move, is just one example of this pattern — and one that IRP researchers have made critical advances on in recent years.
Reflecting the IRP’s groundbreaking research on Parkinson’s disease, in September, IRP senior investigators Ellen Sidransky, M.D., and Andrew Singleton, Ph.D., were awarded the prestigious Breakthrough Prize for their research on the genetic causes of the illness. The world’s largest scientific award, the Breakthrough Prize honors “transformative advances toward understanding living systems and extending human life.” Each year, one award in the Life Sciences category is reserved for research on Parkinson’s and other neurodegenerative disorders. Dr. Sidransky and Dr. Singleton, along with a third Parkinson’s researcher, Thomas Gasser, M.D., Ph.D., at the University of Tübingen in Germany, will share the $3 million prize.
Dr. Singleton and Dr. Sidransky each discovered a genetic cause of the neurological disease, which affects more than 500,000 Americans. We spoke with them to learn more about their discoveries.
One Mutation, Two Diseases
Dr. Ellen Sidransky never set out to find a gene for Parkinson’s disease. As a pediatrician and researcher at NIH’s National Human Genome Research Institute (NHGRI), she was interested in Gaucher disease, a rare genetic disorder that causes cellular garbage to pile up instead of getting recycled or thrown out. However, as her research in Gaucher disease advanced, people with Parkinson’s disease, or family histories of it, kept showing up among her patients. She began to suspect there was a connection.
Gaucher disease is a rare, inherited disorder caused by two faulty copies of a gene called GBA1. People with the disease lack the enzyme needed to break down fat molecules called lipids, causing them to build up in organs like the liver and spleen. The illness can also affect the brain, eyes, lungs, and bones.
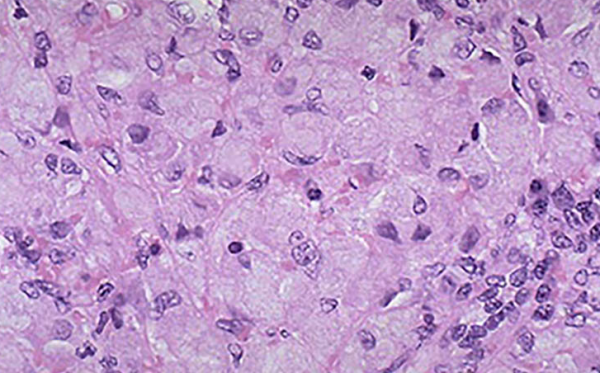
Enlarged cells stuffed with lipids in the spleen of a person with Gaucher disease.
Of the many patients with Gaucher disease Dr. Sidransky treated at the NIH Clinical Center, one woman in her 40s stood out. She had also developed early-onset Parkinson’s disease and donated her body to NIH upon her death. Dr. Sidransky began asking colleagues if they ever saw patients with both diseases. The answer from many was “yes.”
“Every clinic seemed to have a few cases, so I set up some collaborations and collected DNA from about 20 patients,” Dr. Sidransky says. After publishing a series of case studies about these patients, a doctor in Boston found her paper and offered to send her tissue samples from a patient with both Gaucher and Parkinson’s, along with tissue from other Parkinson’s patients for comparison.
“When the three sample sets arrived, the labels were blurred, so I said, ‘Let’s sequence the gene in all three and see if we can identify the one with Gaucher,’” Dr. Sidransky recalls. When the results came back, the patient with Gaucher disease had two mutated copies of the GBA1 gene. The surprise, Dr. Sidransky says, was that the other two samples — from people with Parkinson’s disease but not Gaucher disease — showed the individuals still had one mutated copy of the gene, meaning they could pass the genetic change on to their children without exhibiting symptoms of Gaucher disease themselves.
“This really blew us away,” Dr. Sidransky says. “I immediately reached out to all kinds of brain tissue banks for samples from patients with Parkinson’s disease. Out of 57 patients, 12 had mutations in the Gaucher gene, which was an awful lot.”
That observation spurred her to spearhead a large international collaboration that included 10,000 patients and healthy individuals. The study determined that patients with Parkinson’s disease were at least five times more likely to have a mutation in the GBA1 gene than people without Parkinson’s.1
The link between GBA1 mutations and Parkinson’s disease risk may point to a shared treatment for at least some cases of Parkinson’s disease. In Gaucher disease, patients are treated with infusions of the missing enzyme that they need to break down fats, but this treatment cannot enter the brain. Dr. Sidransky is currently working with colleagues at the National Center for Translational Sciences (NCATS) to identify a therapy that could pump up low levels of that fat-busting enzyme in patients with Parkinson’s.
“If we come up with a treatment and assume that maybe 10 percent of patients with Parkinson’s disease have the GBA1 mutation, the drugs we develop could impact those patients,” Dr. Sidransky says. “In addition, there is evidence that even patients with run-of-the-mill Parkinson’s without this mutation also have lower than normal levels of the enzyme, so it’s theoretically possible that any drug that raises its level could help those patients as well.”
Dr. Sidransky’s laboratory has also been developing better tools for identifying appropriate drugs and building better cellular models of both Gaucher and Parkinson’s disease, including neurons grown from induced pluripotent stem cells that have GBA1 mutations.2 Her lab is also hard at work creating better tests to measure the levels of enzyme activity in cells and patients. In addition, they have been recruiting patients for clinical trials, including pairs of siblings in which both have Gaucher disease but only one has Parkinson’s. Ultimately, Dr. Sidransky’s team hopes its research will identify other factors that may raise or lower the risk of developing Parkinson’s disease.
“We study rare diseases to help the patients with rare diseases and because they’re fascinating,” says Dr. Sidransky, “but we also study them because they can sometimes give us a really unique perspective into medicine in general.”
Treating Parkinson’s at the Genetic Roots

Dr. Andrew Singleton has been studying genetic changes that contribute to Parkinson’s disease for more than 20 years.
For Dr. Andrew Singleton, Parkinson’s disease represents an intriguing puzzle that underlies so much of biology. He is using genetic science to understand why a single disease can look so different in different patients, even those who carry the same contributing mutation. He began tackling this question at the start of his career, working in the lab of John Hardy, Ph.D., first at the Mayo Clinic in Florida and then when they came together to NIH’s National Institute on Aging (NIA).
“I thought Parkinson’s disease was an untapped area,” Dr. Singleton recalls. “The idea of genetics is to understand disease at its most biological basis. If we can find a gene that imports risk for a disease or causes a disease, that’s a starting point for understanding the disease at the molecular level. Of course, once you understand the process, you can develop therapeutics against it.”
Working in Dr. Hardy’s lab, Dr. Singleton and his colleagues at NIH and several universities around the world began sequencing gene after gene to find the ones contributing to Parkinson’s disease. They ultimately found several genes that were involved in the illness, but one stood out as unusual: the LRRK2 gene, which codes for proteins involved in neuron development.3 Mutations or damage to this gene raise the risk of developing Parkinson’s disease by causing it to produce an over-active form of a molecular switch called a kinase, which turns biological activity on and off. This makes it a promising target for drug therapy.
“Before this discovery, therapeutics for Parkinson’s disease really focused on treating symptoms, but that’s never going to stop the disease,” Dr. Singleton says. “Here, the idea is to understand this disease at the molecular level and then develop therapeutics that will actually stop it.”
Since Dr. Singleton and his labmates linked LRRK2 to Parkinson’s disease in 2004, mutated versions of the gene have been confirmed as the most common cause of inherited forms of Parkinson’s disease, as well as an important risk factor for other forms of the disease. Now, he is building on that work by continuing to explore the biological basis of Parkinson’s disease. This work complements, and promises to be enhanced by, related efforts within NIH’s Intramural Center for Alzheimer’s and Related Dementias (CARD), for which Dr. Singleton serves as Director. Meanwhile, Dr. Singleton is keen to see the results of an ongoing phase III clinical trial being run by pharmaceutical company Denali Therapeutics, which is testing a drug that inhibits the activity of the kinase produced by LRRK2.

NIH’s Intramural Center for Alzheimer’s and Related Dementias resides in Building T44 on the main NIH campus in Bethesda, Maryland.
“The LRRK2 discovery has been incredibly influential,” Dr. Singleton says.
Indeed, Dr. Singleton’s work has spurred researchers around the world to study LRRK2. When he first began to search for and sequence the gene, Dr. Singleton’s small, international team was unusual in a field full of individual gene-hunters. Today, however, work on the genetics of Parkinson’s disease is becoming much less competitive and much more collaborative.
“We're competing collectively with the disease rather than with each other,” he says.
Dr. Singleton now leads a large, international collaboration called the Global Parkinson's Genetics Program, which is collecting genetic samples from 200,000 people around the world. The researchers involved in the project hope to identify more genetic factors that might influence inherited forms of Parkinson’s disease and offer additional treatment targets.
“I think it’s particularly interesting to start to put together networks and pathways that are involved in the disease process,” Dr. Singleton says. “The same mutation and the same disease can be so different. It’s still early days in this space.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
References:
[1] Sidransky E, Nalls MA, Aasly JO, Aharon-Peretz J, Annesi G, Barbosa ER, Bar-Shira A, Berg D, Bras J, Brice A, Chen CM, Clark LN, Condroyer C, De Marco EV, Dürr A, Eblan MJ, Fahn S, Farrer MJ, Fung HC, Gan-Or Z, Gasser T, Gershoni-Baruch R, Giladi N, Griffith A, Gurevich T, Januario C, Kropp P, Lang AE, Lee-Chen GJ, Lesage S, Marder K, Mata IF, Mirelman A, Mitsui J, Mizuta I, Nicoletti G, Oliveira C, Ottman R, Orr-Urtreger A, Pereira LV, Quattrone A, Rogaeva E, Rolfs A, Rosenbaum H, Rozenberg R, Samii A, Samaddar T, Schulte C, Sharma M, Singleton A, Spitz M, Tan EK, Tayebi N, Toda T, Troiano AR, Tsuji S, Wittstock M, Wolfsberg TG, Wu YR, Zabetian CP, Zhao Y, Ziegler SG. Multicenter analysis of glucocerebrosidase mutations in Parkinson's disease. N Engl J Med. 2009 Oct 22;361(17):1651-61. doi: 10.1056/NEJMoa0901281.
[2] Aflaki E, Borger DK, Moaven N, Stubblefield BK, Rogers SA, Patnaik S, Schoenen FJ, Westbroek W, Zheng W, Sullivan P, Fujiwara H, Sidhu R, Khaliq ZM, Lopez GJ, Goldstein DS, Ory DS, Marugan J, Sidransky E. A New Glucocerebrosidase Chaperone Reduces α-Synuclein and Glycolipid Levels in iPSC-Derived Dopaminergic Neurons from Patients with Gaucher Disease and Parkinsonism. J Neurosci. 2016;36(28):7441-52. doi: 10.1523/JNEUROSCI.0636-16.2016.
[3] [Paisán-Ruı́z C, Jain S, Evans E, Gilks WP, Simón J, van der Brug M, López de Munain A, Aparicio S, Martinez Gil A, Khan N, Johnson J, Ruiz Martinez J, Nicholl D, Marti Carrera I, Saénz Peňa A, de Silva R, Lees A, Martí-Massó JF, Pérez-Tur J, Wood NW, Singlton AB. Cloning of the Gene Containing Mutations that Cause PARK8-Linked Parkinson's Disease. Neuron. 2004; 44(4): 595-600. doi:10.1016/j.neuron.2004.10.023.
Related Blog Posts
This page was last updated on Monday, November 13, 2023
