Understanding the Foundations of Immune Defenses
IRP’s Mary Carrington Elected to the American Academy of Arts and Sciences for Insights Into Immune System Variations

Dr. Mary Carrington was recently elected to the American Academy of Arts and Sciences for her discoveries about the ways a specific set of genes influences the immune system.
Some people never seem to get sick, while others catch a new bug of some sort every other week. Humans are immensely variable both in their capacity to shrug off illness and in the ways their bodies respond to medical treatments. IRP senior investigator Mary Carrington, Ph.D., has spent her entire career exploring the biological roots of these differences, and the discoveries she has made earned her election to the American Academy of Arts and Sciences earlier this year.
As the head of the HLA Immunogenetics Section in the Laboratory of Integrative Cancer Immunology at the National Cancer Institute (NCI) and a Senior Principal Scientist within the federally-funded Frederick National Laboratory for Cancer Research, Dr. Carrington is focused on identifying genetic variations that influence people’s resistance to different diseases. She and her colleagues are doing this by studying genes called human leukocyte antigens (HLA), which code for proteins that help the immune system distinguish between our own cells and foreign substances that have entered our bodies, such as viruses, bacteria, or transplanted organs.
“There is huge variation in HLA genes across the human population,” Dr. Carrington explains. “These genes are really important in determining how and whether our immune system recognizes a tumor cell or a virally infected cell.”
While HLAs are vital for proper functioning of the immune system, they can be a double-edged sword. On the positive side, they help trigger an immune response to infections and tumors. However, they can also cause the immune system to overreact and harm the body, triggering autoimmune disease. Furthermore, when HLA genes are mismatched between donors and recipients of bone marrow or stem cell transplants, it can lead to life-threatening conditions like graft versus host disease, in which immune cells produced by the transplanted tissue attack the recipient’s body.
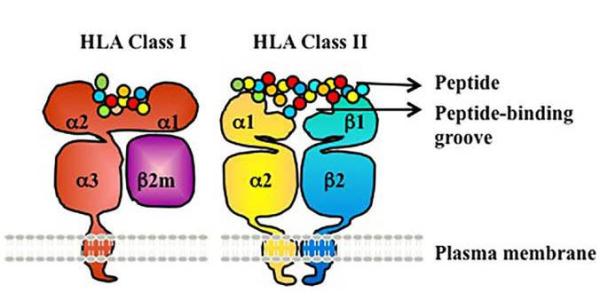
HLA proteins (red, purple, yellow, blue) display peptide molecules (small circles) from inside the cell to help immune cells find cancerous or infected cells. Image credit: Frontiers in Immunology. Feb 2016. https://doi.org/10.3389/fimmu.2016.00030. CC BY 4.0.
Consequently, identifying both the useful and harmful effects of HLA variation can help doctors determine the best course of treatment for a given patient. For example, testing for certain types of HLA can help determine which patients will react well to a specific medication and which ones may see no improvement or even experience harmful effects. A class of cancer drugs called checkpoint inhibitors, for instance, works by curtailing tumors’ ability to hide from the immune system, but only some patients benefit from these drugs.
“We think that the HLA genes determine to some extent how well people respond to these drugs,” Dr. Carrington says. “We’re looking for biomarkers, or physical indicators, that will help us determine whether a patient will do well on these drugs or not. If they’ll do poorly, there’s no point in using them.”
As important as HLA genes are for the effectiveness of cancer immunotherapies, Dr. Carrington has also studied other genes and illnesses over the course of her career. In the late 1990s, for example, Dr. Carrington and her colleagues identified a mutation in a gene called CCR5 that affects people’s susceptibility to HIV infection. The gene codes for a receptor that sits on the outside of cells, and HIV must bind to this receptor to infect the cell. However, if a person inherits a particular mutation in the gene from both parents, his or her cells cannot make the receptor protein, rendering them resistant to HIV infection. After Dr. Carrington and her colleagues made this discovery, scientists developed a drug that blocks the CCR5 receptor in people with HIV in order to curtail the virus’ ability to replicate within their bodies.
“This is just a simple example of a mutation that confers protection because it’s a receptor for a virus,” Dr. Carrington says. “That’s the type of thing we love to figure out.”
More recently, Dr. Carrington has been focusing on the way HLA influences the outcomes of stem cell and bone marrow transplants. If the cells or blood transferred from a donor to a patient have incompatible variation in HLA genes, the transplanted tissue can attack the recipient’s cells, harming his or her health rather than providing a life-saving treatment. However, if doctors can find a donor and a recipient whose HLA profiles are identical or similar in specific ways, the transplant can take place successfully. The challenge, especially when the patient has no HLA-compatible relatives, is to determine which donors and recipients are an acceptable match.
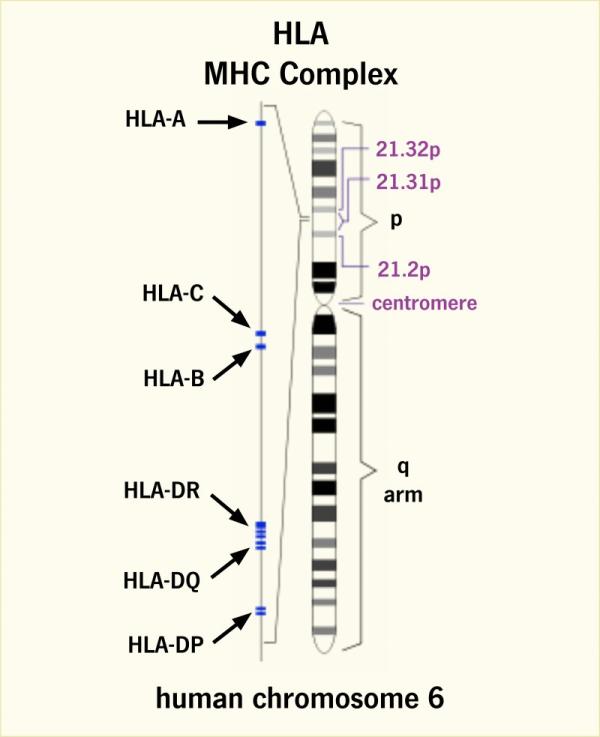
Our HLA genes are located in a region of chromosome 6 called the major histocompatibility complex (MHC).
In an attempt to answer this question, Dr. Carrington and her colleagues are currently collaborating with researchers at the Fred Hutchinson Cancer Research Center in Seattle, Washington, as well as clinicians at transplant centers around the world, to study a large group of people who received bone marrow transplants to treat blood cancers. Dr. Carrington and her collaborators compared the HLA genes and health outcomes of each patient and his or her bone marrow donor to determine which genetic mismatches were safe and which ones caused harm.
“A lot of people who need a bone marrow transplant do not have a matched sibling, so they have to rely on unrelated donors,” Dr. Carrington says. “Now we know people who have specific mismatches at a certain HLA gene are very likely to be okay even though it’s a mismatch, whereas we can avoid certain mismatches that we know won’t work. It has an immediate clinical application.”
Today, Dr. Carrington is expanding her research on cancer immunology, building up collaborations with research centers around the country and putting together large groups of study participants. At the same time, she is continuing her work on HIV/AIDS, a topic that fascinates her because HLA genes have such a large impact on HIV infection and resistance.
“It’s pretty endless, the level at which these genes are involved in human disease, so I’ll be busy for the rest of my career,” says Dr. Carrington. "It’s really wonderful when your work is also your hobby.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
Related Blog Posts
- HHS Awards Recognize IRP Cancer Researchers
- Postdoc Profile: From Bench to Bedside and Back Again
- Breakthrough Treatment Brings Hope to Children with Tumor Disorder
- IRP’s Luigi Notarangelo Elected to National Academy of Medicine
- IRP’s Marston Linehan Receives HHS Secretary’s Award for Distinguished Service
This page was last updated on Monday, January 29, 2024
