Breakthrough Treatment Brings Hope to Children with Tumor Disorder
Brigitte Widemann Recognized for Pioneering Work on Debilitating Disease

Dr. Brigitte C. Widemann was named a finalist for the 2021 Samuel J. Heyman Service to America Medal for developing a breakthrough treatment for a disease that causes inoperable tumors in children.
Getting diagnosed with a serious illness as an adult can be devastating, so one can hardly imagine the impact of receiving such news as a child, particularly when the disease has no good treatments. Until recently, this was the case for many children with the potentially severe and frequently disfiguring condition neurofibromatosis type 1 (NF1). However, pioneering research led by IRP senior investigator Brigitte C. Widemann, M.D., led to the first-ever drug approved by the U.S. Food and Drug Administration (FDA) to treat the condition. For this groundbreaking work, Dr. Widemann, her IRP research team, and her collaborators outside NIH were named as finalists for the 2020 Samuel J. Heyman Service to America Medals, also known as the ‘Sammies,’ an award that honors exceptional work by government employees.
NF1 is a genetic disorder that causes non-cancerous tumors to grow on nerves throughout the body. It affects an estimated 1 in 3,000 children and young adults around the world. Although not all people with the mutation responsible for NF1 develop tumors, called plexiform neurofibromas, in the 20 to 50 percent who do, the effects can be debilitating. Even though they are benign, the tumors cause pain and disfigurement. In addition, depending on where the tumors grow, they can affect vision, motor coordination, bladder and bowel function, and even breathing. Some of these tumors can also ultimately become cancerous.
What’s more, because of their location, tangled among delicate nerves, it is often impossible to operate on these tumors without the risk of nerve damage. Moreover, those that are surgically removed often grow back.
“Kids develop these tumors very early in life,” Dr. Widemann explains. “Many patients are born with the tumors already in their bodies. They grow quite relentlessly.”
Dr. Widemann’s work focuses on clinical research to develop therapies for childhood cancers and tumor-causing genetic disorders for which there are no effective treatments. In addition to her role as chief of the Pediatric Oncology Branch at the National Cancer Institute (NCI), she serves as the principal investigator for several clinical trials coordinated by herself or various consortia that conduct early-stage clinical trials, often at the NIH Clinical Center, with the goal of developing drugs that might bring relief to patients with these often devastating diseases and cancers.
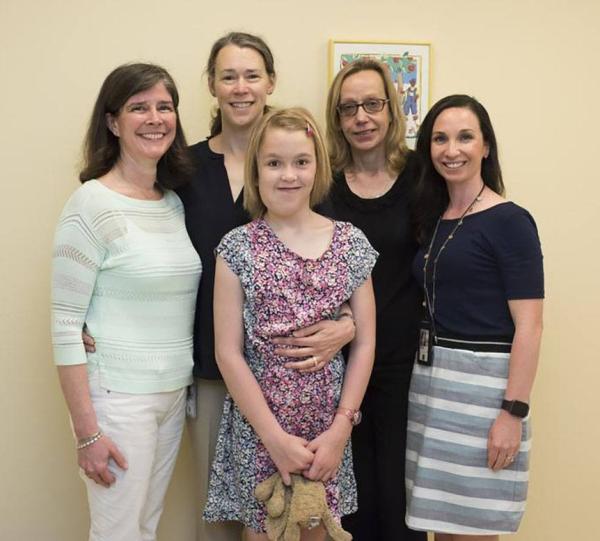
A young patient with NF1 who was treated at NCI gathers with her mother and members of Dr. Widemann’s IRP team.
NF1 is one such genetic tumor predisposition syndrome that has eluded effective treatment. Past studies had shown that the related mutation disrupts an important signaling pathway called RAS, which controls normal cell growth and division. Mutations can keep the RAS pathway permanently switched on, so it never stops telling cells to divide and grow. As a result, tumors develop. Dr. Widemann and her colleagues thought that targeted drugs that could inhibit or turn off the RAS pathway could slow or even stop progression of NF1 and possibly other tumor predisposition conditions.
“Identifying how we can effectively block this RAS pathway with small molecules has been the holy grail for us,” Dr. Widemann says.
After conducting a series of largely unsuccessful early-stage clinical trials with various therapies for children and young adults with NF1 and inoperable plexiform neurofibromas, Dr. Widemann and her team turned to a drug called selumetinib, which inhibits the activity of an enzyme involved in the RAS pathway. Although selumetinib had failed in a clinical trial for treating metastatic breast cancer, it showed promising results for NF1 in a Phase I clinical trial conducted by Dr. Widemann’s group.
“For the first time ever, we were able to show that in the majority of patients, not only did the tumor stop growing, but there was actually shrinkage,” Dr. Widemann says. “That was really an amazing finding after many years of work, not only here in the Intramural program, but also with colleagues at our other study sites.”
In partnership with colleagues in NCI’s Cancer Therapy Evaluation Program, collaborators outside NIH, and the pharmaceutical company AstraZeneca — which developed selumetinib — Dr. Widemann and her team subsequently ran a second clinical trial with the intention of applying for FDA approval if results were positive. The trial was ultimately a success.
In preparation to apply for FDA approval, Dr. Widemann’s team also utilized data from a ‘natural history’ study of NF1, which provided a baseline understanding of how plexiform neurofibromas progress without treatment. Researchers could then compare that baseline with the rates of tumor growth seen in clinical trials. That long-term observational study followed 259 children over 10 years, and comparing tumor growth in children treated with selumetinib to children of the same age in the natural history study revealed that selumetinib not only shrank existing tumors, but also delayed the growth of new ones. With the results of the NF1 natural history study in hand, the FDA approved selumetinib as a treatment for children with NF1 and inoperable symptomatic plexiform neurofibromas in April 2020, with approval in Brazil and Europe following in 2021.
Meet Autumn, one of the children whose participation in Dr. Widemann's clinical trials led to the FDA approval of selumetinib for NF1. She stayed at the Children's Inn at NIH while receiving treatment at the NIH Clinical Center.
Of course, this thrilling result could not have happened without the help of Dr. Widemann’s patients, many of whom she has watched grow to adulthood. Working with them is one of the most inspiring aspects of her work, she says, and she counts them as important members of her clinical trial team, along with the doctors, nurses, and patient coordinators.
“They come seeking better treatment and sometimes cures, but they also come because they want to advance better therapies,” Dr. Widemann says. “They make contributions and are incredibly inspiring.”
Dr. Widemann did not see foresee herself leading such important research at NIH when she first arrived there as a clinical fellow in 1992; on the contrary, she didn’t expect to stay all that long. However, she quickly came to appreciate the IRP’s dedicated, collaborative research community and realized she would have a unique opportunity at NIH to focus on helping patients with challenging and neglected cancers.
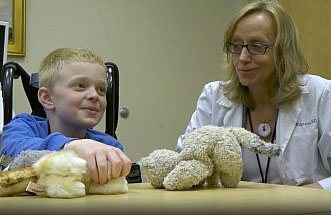
Dr. Brigitte Widemann with a young patient who received selumetinib for NF1 at NIH.
“Coming to NIH for the fellowship really changed my life,” Dr. Widemann says. “I was initially planning to be at the NIH for three years, the duration of the fellowship, but I became so engaged in my research and in designing clinical trials for children that I didn’t want to stop. I enjoyed it so much that I stayed on, and I can say this is the best place to do this type of work.”
Today, she is continuing that work with her colleagues in the Pediatric Oncology Branch. Together, they are moving forward with clinical trials to see if selumetinib is effective in adults with NF1, as well as looking for an effective therapy for patients whose NF1 tumors become aggressively cancerous. At the same time, Dr. Widemann’s IRP team is applying lessons learned from their work with NF1 to other rare cancers, such as medullary thyroid cancer and other rare tumors. After years of working so closely with researchers both within and outside NIH, she is happy see the group’s efforts recognized by the Sammy awards.
“Sometimes I feel so humbled, because the Service to America medal is awarded to many brilliant people who make such important contributions,” Dr. Widemann says. “On the other hand, I'm really excited and grateful and honored. This doesn’t only reflect my work — it reflects that of the whole team. I'm really proud of the team that has worked and made this advance.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
Related Blog Posts
This page was last updated on Monday, January 29, 2024
