Sugar Molecule Could Resolve Rare Diseases
IRP Research Hastens Development of First Treatment for Genetic Muscle Condition
Patients with a rare disease called GNE myopathy are closer than ever to having an effective treatment thanks to the work of IRP researchers led by Dr. Marjan Huizing.
An old medical adage warns doctors that when they hear hoofbeats, they should first think of horses, not zebras. After all, when someone comes into the hospital with a cough, the most likely explanation is something mundane like the flu. However, some patients truly are medical zebras, affected by a disease that afflicts very few others.
IRP senior investigator Marjan Huizing, Ph.D., has learned quite a bit about those zebras since arriving at the National Human Genome Research Institute (NHGRI) as a postdoctoral fellow in 1998. To help commemorate Rare Disease Day today, Dr. Huizing spoke with the “I Am Intramural” blog about her research on an array of ailments linked to a small sugar molecule called sialic acid, some of which are extremely rare.
“Sialic acid is associated with many, if not all, biological processes, including interactions between cells and immune responses,” Dr. Huizing says, “so if there is a defect in how the body processes sialic acid, it can cause disease. In fact, sialic acid defects have been associated with cardiovascular disease, muscular disease, kidney disease, diabetes, sleep disorders, and learning difficulties.”
When Dr. Huizing came to NIH to work with IRP senior investigator William Gahl, M.D., Ph.D., his team was studying genetic mutations that disrupt the creation of sialic acid and are associated with several rare diseases. For example, Dr. Gahl’s team had identified mutations in a gene called GNE that cause individuals to produce too much sialic acid. Other mutations in the gene lead to a lack of sialic acid, which leads to a condition called GNE myopathy with symptoms that include progressive muscle weakness, muscle atrophy, and loss of balance and grip strength. GNE myopathy has been reported in only about 1,500 patients worldwide, but is thought to affect at least twice that many due to underdiagnosis — a typical problem for very rare diseases.
There is currently no treatment for GNE myopathy, but Dr. Huizing hopes her team’s discovery of a simple sugar molecule that the body uses to create sialic acid, called N-acetylmannosamine, or ‘ManNAc’ for short, may change that. When the IRP scientists fed the molecule to mice whose bodies produced too little sialic acid, the animals’ health improved.1
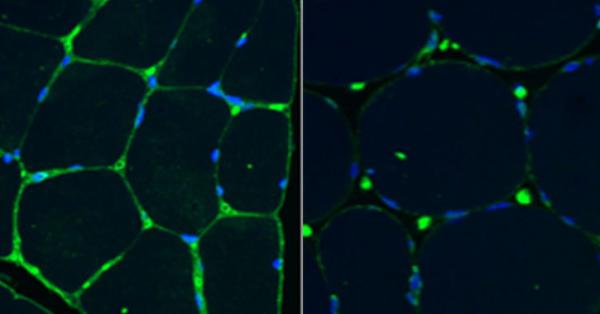
These muscle biopsy slides show sialic acid in green and cell nuclei in blue. Levels of sialic acid are higher in the muscle tissue of a healthy control (left) compared to the muscle of a patient with GNE myopathy (right). Image adapted from: Carrillo, Malicdan, Huizing. Neurotherapeutics (2018), 15900-15914.
“It was a surprise,” Dr. Huizing says. “We could just add it to their drinking water and it increased sialic acid levels. That opened up a lot of possibilities not only for GNE myopathy, but for some other diseases that are associated with abnormal sialic acid production.”
The road to effective treatments for rare diseases is often long and difficult, even when the candidate therapies are promising. This has certainly been the case for ManNAc. Dr. Huizing’s research group patented the compound in 2007 and was fortunate to find a company in New Zealand willing to produce clinical-quality ManNAc for free to use in an initial clinical trial. That’s when her team hit its first roadblock.
“Since ManNAc was a neutral sugar, we thought we could safely test it in patients with a rare disease,” Dr. Huizing says. “In 2007, we had the drug, we had the clinical protocol and the Investigational New Drug application written, and we had the patients ready for a phase 1 clinical trial, but the FDA put the trial on hold pending full toxicology studies. At the time, that would have cost about $1 million.”
Dr. Huizing and her collaborators applied for multiple grants but didn’t qualify. Luckily, just when they were close to giving up, NIH started the Therapeutics for Rare and Neglected Diseases (TRND) program. The program selected the ManNAc trials as a pilot project and performed the needed safety testing on the molecule.

Dr. Marjan Huizing
“The TRND program performed invaluable work that we’re still benefitting from,” Dr. Huizing says.
Dr. Gahl's and Dr. Huizing’s research team was finally able to start clinical trials to test ManNAc in 2014 and continues them to this day.2 Those studies have so far demonstrated that ManNAc is safe, helped the researchers identify the proper dose, and, most importantly, demonstrated the treatment increases sialic acid levels in muscles. Currently, the IRP team is working with a pharmaceutical company to conduct a large clinical trial testing ManNAc’s effectiveness in patients with GNE myopathy.
“We’re 22 years further down the line in research and there’s still no therapy for these patients,” Dr. Huizing says. “That is very typical for a rare disease. We really need to shorten the timeline from discovery in a lab to getting the drug to the patient.”
Meanwhile, Dr. Huizing and her colleagues began thinking about how ManNAc could be used to treat other diseases that stem from defects in sialic acid production, including ailments much more common that GNE myopathy. They had noticed that the mouse model they used in their pre-clinical studies of ManNAc had defects in the filters, called glomeruli, that allow the kidneys to remove toxins from blood, and their glomeruli also had very low levels of sialic acid. While people with GNE myopathy do not have this particular problem, the researchers thought maybe patients with other diseases that do affect the glomeruli could also benefit from receiving ManNAc.
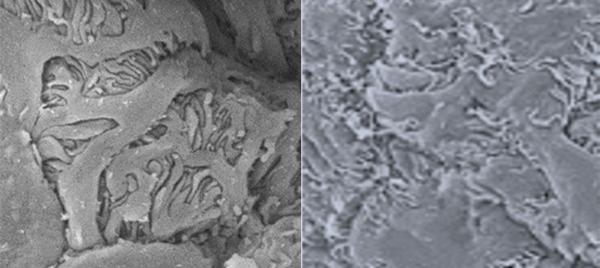
These scanning electron microscope images show mouse kidney glomeruli. The image on the left comes from a healthy mouse, while the image on the right shows fused and flattened glomeruli in a mouse with a disease that reduces sialic acid levels in its glomeruli.
Working with IRP senior investigator Jeffrey Kopp, M.D., they tested tissue samples from the glomeruli of 100 patients with kidney disease and found 70 percent of them had abnormally low levels of sialic acid. A subsequent phase 1 trial showed ManNAc was safe and appeared to improve kidney function in patients with defective glomeruli after only five days of treatment.3 The IRP team is now looking for a pharmaceutical company to take on larger clinical trials.
Dr. Huizing has also begun studying a genetic condition even more rare than GNE myopathy. People with free sialic acid storage disorder, also called Salla disease, lack the transporter necessary to remove sialic acid from their cells. Only about 250 people in the world have been identified with the condition, including a young boy in a family living in New York City. After learning there was no therapy and little research happening, that family brought salic acid researchers from around the world together to find a treatment. The group first met in 2018 and, according to Dr. Huizing, aims to find a therapy within the next five years.

Researchers studying free sialic acid storage diseases met at NIH in September 2022 to discuss their progress and next steps. Such meetings are critical for facilitating the sorts of collaborations needed to development treatments for rare diseases.
Such collaborations, along with the depth and breadth of resources and opportunities available to IRP scientists like Dr. Huizing, are essential for conducting the long-term research required to develop treatments for rare diseases.
“You learn so many new aspects along the way and it's not driven by funding — it comes from your heart,” she says. “It's something that you want to accomplish.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
References:
[1] Galeano B, Klootwijk R, Manoli I, Sun MS, Ciccone C, Darvish D, Starost MF, Zerfas PM, Hoffmann VJ, Hoogstraten-Miller S, Krasnewich DM, Gahl WA, Huizing M. Mutation in the key enzyme of sialic acid biosynthesis causes severe glomerular proteinuria and is rescued by N-acetylmannosamine. J Clin Invest. 2007; 117(6):1585-1594. doi: 10.1172/JCI30954.
[2] Carrillo N, Malicdan MC, Leoyklang P, Shrader JA, Joe G, Slota C, Perreault J, Heiss JD, Class B, Chia- Liu Y, Bradley K, Jodarski C, Ciccone C, Driscoll C, Parks R, Van Wart S, Bayman L, Coffey CS, Quintana M, Berry SM, Huizing M, Gahl WA. Safety and efficacy of N-acetylmannosamine (ManNAc) in patients with GNE myopathy: an open-label phase 2 study. Genetics in Medicine. 2021; 23:2067–2075; doi: 10.1038/s41436-021-01259-x.
[3] Huizing M, Yardeni T, Fuentes F, Malicdan MCV, Leoyklang P, Volkov A, Dekel B, Brede E, Blake J, Powell A, Chatrathi H, Anikster Y, Carrillo N, Gahl WA, Kopp JB. Rationale and Design for a Phase 1 Study of N-Acetylmannosamine for Primary Glomerular Diseases. Kidney Int Rep. 2019; 4:1454–1462. doi: 10.1016/j.ekir.2019.06.012.
Related Blog Posts
This page was last updated on Tuesday, May 23, 2023
