Mislabeled Proteins Linked to Birth Defects
IRP Researchers Discover Genetic Disorder Affecting the Brain and Skull

IRP researchers with a variety of expertise came together to pinpoint the genetic cause of birth defects affecting an infant patient at the NIH Clinical Center, leading to the discovery of a new developmental syndrome.
A baby is born with a birth defect every four and a half minutes in the United States, adding up to one in every 33 babies born each year in this country. While some birth defects can be corrected or treated, many result in life-altering disabilities, and sometimes the child doesn’t survive. In fact, birth defects account for about 20 percent of infant deaths in the U.S. World Birth Defects Day, celebrated on March 3 each year, honors the people and organizations who are working to understand, prevent, and treat birth defects.
One of these organizations is the Undiagnosed Diseases Program (UDP) at NIH, which connects experts across the IRP’s 23 Institutes and Centers in a joint effort to find explanations for the “most puzzling medical cases" referred to the NIH Clinical Center.
One of these cases occurred in 2018, when a family enrolled in the program in search of an explanation for their infant son’s severe birth defects. The family had suffered the loss of two other sons born with similar abnormalities in the brain, skull, and face, and they hoped that genetic sequencing would shed light on the source of the problems. As a result, the UDP brought together a group of IRP experts on genomics and embryonic development to analyze the DNA of the affected baby and several members of the family. The genetic analysis, which focused specifically on the parts of DNA that contain instructions for producing proteins, ultimately led to the discovery of a new genetic syndrome.
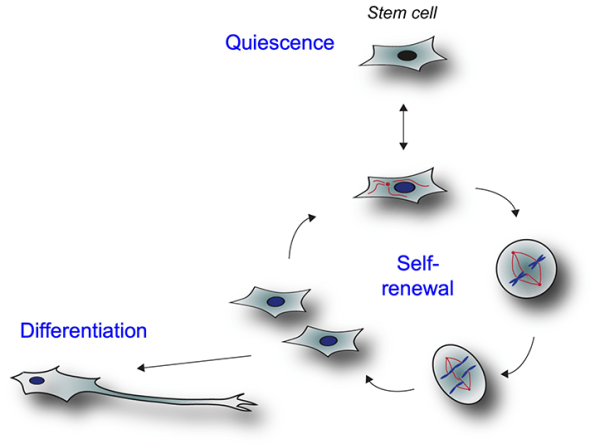
Dr. Werner is an expert on stem cells, which can either ‘self-renew’ to produce more stem cells or ‘differentiate’ to mature into specialized cells like lung, heart, or bone cells.
That team was led by IRP Stadtman Investigator Achim Werner, Ph.D. At the National Institute of Dental and Craniofacial Research (NIDCR), Dr. Werner studies how cells called stem cells develop and evolve into the various types of specialized, mature cells that make up our organs, cardiovascular system, and bones. He is particularly interested in how the brain and skull develop. At the time, he was co-mentoring IRP clinical research fellow David Beck, M.D., Ph.D., who is now an assistant professor at New York University. Dr. Beck was treating the infant boy and brought his young patient’s mysterious case to Dr. Werner and his other mentor, NIH Distinguished Investigator Dan Kastner, M.D., Ph.D., a geneticist working at the National Human Genome Research Institute (NHGRI). Together, they analyzed the family’s genetic data and identified a single suspect gene in the affected baby’s DNA.
“The genetics were quite clear,” Dr. Beck says. “One particular gene called OTUD5 stood out.”
“This gene codes for an enzyme that plays a role in the pathway of ubiquitylation, which is basically a tagging system within the cell to direct what happens to each protein.” Dr. Werner adds.
When cells create the proteins that are the building blocks of our bodies, they need to translate the DNA instructions stored in the cell’s nucleus. This biochemical activity is helped along by special proteins called ‘chromatin remodelers,’ which fold and bundle DNA in the cell’s nucleus. This process exposes sections of DNA to be transcribed into the messenger RNA that is ultimately used to create proteins, while also hiding sections of DNA that aren’t needed.
In ubiquitylation, a small molecular tag called ubiquitin is attached to different proteins like an instruction label. It can direct the cell to destroy the protein or move it to a certain location in the cell, and it can also affect the protein’s activity and promote or prevent interactions with other proteins. When a mutation disrupts ubiquitylation, the instructions can get lost, and the proteins are no longer directed correctly. For this reason, mutations affecting the ubiquitin label are associated with several neurodegenerative disorders, including Alzheimer’s disease, Huntington’s disease, and Parkinson’s disease.
However, the discovery that the OTUD5 gene, which is involved in ubiquitylation, might be responsible for the infant boy’s birth defects was a surprise. Dr. Werner’s team determined that the gene codes for a protein that undoes the ubiquitylation process by slicing off the tags on chromatin remodelers, thereby preventing the remodelers from being destroyed. However, when OTUD5 is mutated, the cell gets rid of the chromatin remodeling proteins, which deprives cells of the correct instructions for normal development and leads to birth defects like those seen in the boy at the NIH Clinical Center.
Dr. Werner and Dr. Beck discuss their research on OTUD5-related birth defects in this NIDCR video.
Those findings, in turn, spurred a collaboration between Dr. Werner’s team and the lab of IRP Stadtman investigator Pedro Rocha, Ph.D., an expert in chromatin remodeling at the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). Together, the two research groups used a special sequencing technique to identify genes that are not properly activated when OTUD5 does not work correctly in a person’s cells.
“OTUD5 had never been linked to embryonic development,” says Dr. Werner, “so we had to dive into the unknown and establish new model systems to determine whether this gene was critical to this function.”

Dr. Achim Werner
Once Dr. Werner’s team had identified the OTUD5 mutation, they connected with other clinicians and researchers around the world through a website called geneMatcher. Using this website, they were able to find more patients with mutations in OTUD5. They reached out to those families and physicians and invited them to participate in their study.
“More than 20 patients have been identified now — more than twice the number compared to when we published our study in 2021,” says Dr. Werner. “They all have mutations in the OTUD5 gene, but the errors are located in different areas along the gene,” variations which may impact the symptoms and severity of the syndrome, although that possibility requires further study.
“I think the major accomplishment in this study was two-fold,” Dr. Werner adds. “We characterized the gene and confirmed that it is important for early embryonic development and were also able to go back to the family with a diagnosis.”
Dr. Werner’s team named the previously unknown condition responsible for the boy’s birth defects LINKED syndrome, which stands for “LINKage-specific deubiquitylation deficiency–induced Embryonic Defects.” LINKED affects only male patients and is marked by physical deformities of the face and skull, as well as the genitourinary, cardiac, and central nervous systems. Some patients are also born with extra fingers and toes. In addition, LINKED causes developmental delays, meaning affected children experience cognitive and physical problems. It can even be fatal in infancy or lead to death relatively early in life, although Dr. Werner says several patients have been identified who are in their 40s. Unfortunately, those patients have experienced severe cognitive and movement challenges.
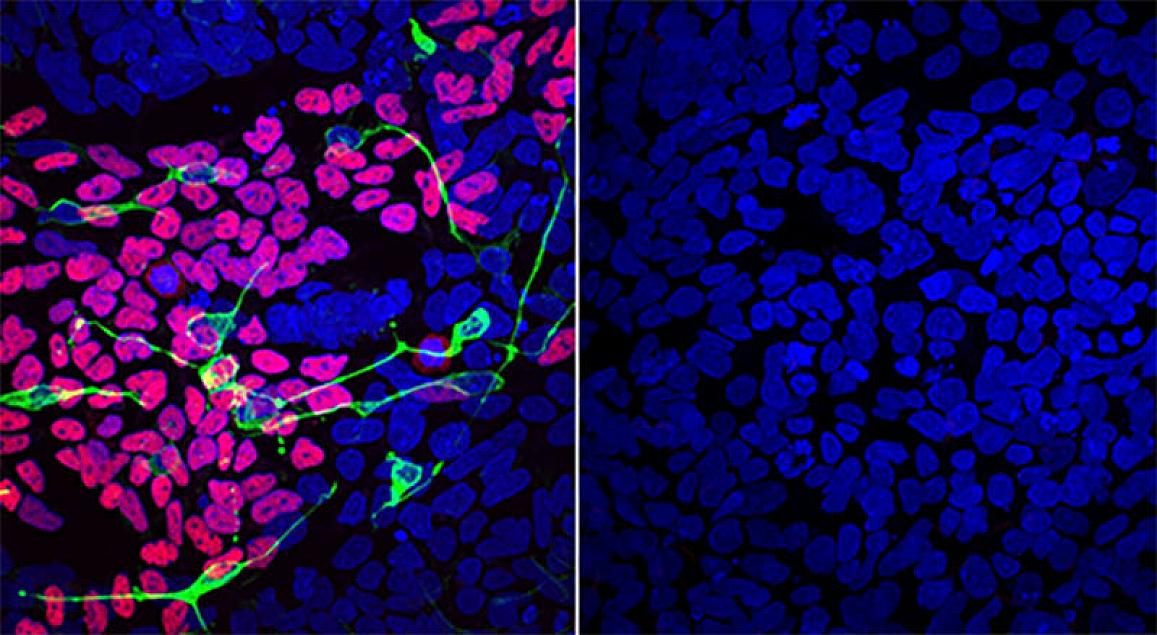
Image courtesy of the Werner Lab, NIDCR
Compared to a disease-free mother (left), differentiated LINKED patient cells (right) lack markers of normal development of the brain, spinal cord, and craniofacial skeleton (pink, green and yellow).
Today, Dr. Werner’s team is expanding its studies to further understand the process of how adding and removing ubiquitin tags from chromatin remodelers contributes to normal development. In particular, they are intrigued by the observation that many of the chromatin remodelers that are controlled by OTUD5 also lead to birth defects when the remodelers themselves are mutated.
“You get developmental diseases that are distinct but have overlapping features,” Dr. Werner says. “That’s something we’re really excited about because adding and removing these ubiquitin tags from these chromatin remodeling proteins seems to be a central pathway in embryonic development.”
“I’m excited about this approach and the opportunity for our lab to collaborate with clinicians,” Dr. Werner continues. “I’m not an M.D., so I never get to see the patients. While I’m fascinated by figuring out how these mechanisms work in the cell, now we can work together to find strategies to correct the problem. That’s what keeps me up at night and here on weekends.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
References:
[1] Linkage-specific deubiquitylation by OTUD5 defines an embryonic pathway intolerant to genomic variation. Beck DB, Basar MA, Asmar AJ, Thompson JJ, Oda H, Uehara DT, Saida K, Pajusalu S, Talvik I, D’Souza P, Bodurtha J, My W, Barañano KW, Miyake N, Wang R, Kempers M, Tamada T, Nishimura Y, Okada S, Kosho T, Dale R, Mitra A, Macnamara E, Undiagnosed Diseases Network; Matsumoto N, Inawaza J, Walkiewicz M, Õunap K, Tifft CJ, Aksentijevich I, Kastner DL, Rocha PP, Werner A. Sci Adv. 2021 Jan 20;7(4):eabe2116. doi: 10.1126/sciadv.abe2116.
Related Blog Posts
This page was last updated on Tuesday, January 30, 2024
