Overturning the Orthodoxy About the Brain’s Stress Chemical
IRP Researchers Discover Unexpected Stress-Blunting Effects of Some Neurons

Recent findings by IRP researchers are changing long-held beliefs about how a particular chemical influences the brain’s response to stress.
The past few years have not been easy for anyone. With world events like the COVID-19 pandemic and the war in Ukraine causing everyone to worry, it’s no surprise that during this April’s annual Stress Awareness Month observance, so many people experienced high levels of stress and anxiety. While stress management techniques and talk therapy may help some people, nearly 10 million Americans need prescription anti-anxiety drugs to quell those feelings.
One important target for anti-anxiety medications is norepinephrine, a chemical released by certain neurons in the brain. Norepinephrine — also known as noradrenaline — has traditionally been considered to be a ‘stress chemical’ that triggers anxiety. However, drugs designed to target the neurons that produce it don’t always work as predicted. That’s why IRP senior investigator Patricia Jensen, Ph.D., and her colleagues in the Developmental Neurobiology Group at the National Institute of Environmental Health Sciences (NIEHS) are delving deep into the mouse brain to better understand these neurons and what exactly they do.
Most research on the ‘noradrenergic’ neurons that release norepinephrine concentrates on the cells located in a part of the brainstem called the locus coeruleus. However, these neurons also exist in other parts of the brainstem, and these additional noradrenergic neurons differ from those in the locus coeruleus in how they connect to other brain areas and how they respond to stressors. In fact, there have been hints in some studies that some of these neurons actually blunt the stress response rather than promote it as their cousins in the locus coeruleus do.
“If you go into the scientific literature, you’ll find all these hints that sometimes we get a different response,” says Dr. Jensen.
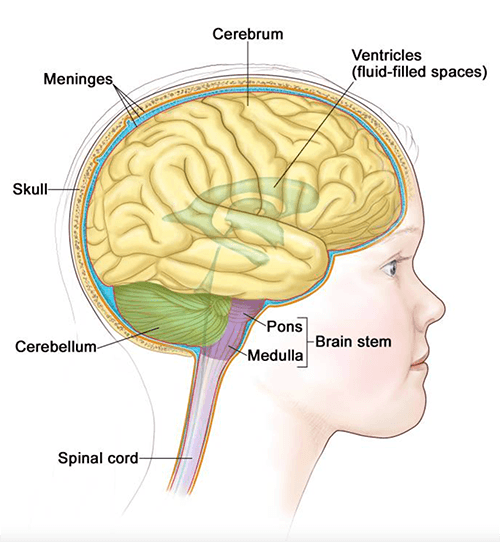
Image credit: Terese Winslow
The noradrenergic neurons Dr. Jensen identified reside in the pons and medulla, and some of them project to the prefrontal cortex, which is part of the brain’s cerebrum.
In order to shed light on those counter-intuitive findings, Dr. Jensen’s team examined the brains of mice to better characterize the distinctive features of noradrenergic neurons located outside the locus coeruleus. That study, published in 2013, identified four genetically distinct populations of noradrenergic neurons in mice located in parts of the brainstem other than the locus coeruleus, some of which appeared to function differently from the ones in the locus coeruleus.1 What’s more, one set of these neurons connected to areas in the prefrontal cortex, a part of the brain that plays a key role in many aspects of behavior, including planning and decision making. In those neurons, a gene called Hoxb1 was highly active while the animals’ brains were developing, but not in adult mice.
The 2013 study overturned the orthodoxy that the noradrenergic neurons in the locus coeruleus are the only ones that send signals to the prefrontal cortex. It also prompted Yu-Wei Chen, Ph.D., who was then a postdoctoral fellow at the National Institute on Drug Abuse (NIDA), to get in touch with Dr. Jensen. Dr. Chen thought that perhaps it was the noradrenergic neurons in which Hoxb1 was active during development that were responsible for the inconsistent results of prior studies examining norepinephrine’s role in the stress response.
Ultimately, Dr. Chen’s curiosity led her to move to NIEHS to join Dr. Jensen’s team, and together they made some important discoveries about that set of noradrenergic neurons. In a study published in 2019, Dr. Jensen’s lab showed that those neurons send signals to areas in the brain that are involved in central autonomic control — the part of the body’s nervous system that controls bodily functions we don’t need to think about, like breathing, blood pressure, and heart rate. What’s more, in mice, those neurons had an antidepressant-like effect when stimulated: they promoted better coping responses to stressors like being placed in water and they decreased the animals’ anxious behaviors.2

Dr. Patricia Jensen
“The 2019 paper was really exciting because we found this other noradrenergic population where we get the complete opposite response to stress,” Dr. Jensen says.
In order to continue studying those neurons, Dr. Jensen’s team will have to overcome some significant hurdles. The cells are difficult to study because they’re scattered around the brain and the Hoxb1 gene isn’t active in the brains of adult mice, which makes it difficult to figure out what might happen if their influence were tamped down in fully grown animals.
“We couldn’t yet do the appropriate follow-up experiments,” Dr. Jensen explained. “Our prediction was that by inhibiting those neurons and then exposing the mouse to a physical stressor, the stress response would be higher because we think this is an opposing system to the one in the locus coeruleus.”
Since then, Dr. Jensen and her research team have successfully bred mice in which they can experimentally manipulate those stress-blunting noradrenergic neurons. These mice provide her team with a way to turn off those nerve cells in the brains of adult mice, allowing them at last to test their theories.
“This is when the most exciting science happens, when you pick up on some little thing that’s different and you ask, ‘What’s this all about?’” Dr. Jensen reflects. “We try to encourage our students and trainees not to ignore what they’re seeing. Do the experiment and replicate it, but if you keep seeing the same thing, don’t worry if the dogma says the opposite.”
References:
[1] Robertson S, Plummer NW, de Marchena J, Jensen P. Developmental origins of central norepinephrine neuron diversity. Nat Neurosci. 2013;16(8):1016-23. doi: 10.1038/nn.3458.
[2] Chen YW, Das M, Oyarzabal EA, Cheng Q, Plummer NW, Smith KG, Jones GK, Malawsky D, Yakel JL, Shih YI, Jensen P. Genetic identification of a population of noradrenergic neurons implicated in attenuation of stress-related responses. Mol. Psychiatry. 2019 May; 24(5):710-725. doi: 10.1038/s41380-018-0245-8.
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
Related Blog Posts
This page was last updated on Wednesday, May 24, 2023
