Mouse Study Links Anxiety to Neuronal Power Failure
Chronic Stress Diminishes Energy Production in the Brain

Experiments by IRP researchers suggest that stress-induced problems with cells’ energy-producing mitochondria might contribute to some anxiety disorders.
When power lines come down and the electricity shuts off, it’s understandably a worrying situation. As it turns out, people may become anxious not just when their homes are cut off from energy, but also when their brains find themselves short on power, according to recent IRP research done in mice.1
While the misfortune of a blackout is temporary, many people experience chronic stress that bothers them continuously. In some individuals, repetitive stressors can contribute to the development of debilitating anxiety that interferes with everyday life. Intriguingly, past research has found evidence that problems with the biological batteries that power our cells, called mitochondria, might be involved in anxiety disorders, as well as some other psychiatric illnesses.
“Mitochondria produce 90 percent of the energy used by our cells,” notes IRP senior investigator Zheng Li, Ph.D., the new study’s senior author. “The brain is heavily dependent on that energy — the brain is something like two percent of our body weight, but uses 20 percent of the energy we consume — so it’s intuitive to think that if mitochondria are messed up and we don’t have enough energy, that will be detrimental to our cognitive processes, our ability to regulate our emotions, and our daily behavior.”
However, it remains unclear whether abnormal mitochondria cause psychiatric ailments or are a consequence of them. In her lab’s new study, Dr. Li set out to answer that question, as well as demystify the biological mechanism linking mitochondrial dysfunction to anxiety.
Mitochondria like those pictured here produce the energy that neurons need to send electrical signals.
Dr. Li’s experiments utilized a mouse model of chronic stress called chronic social defeat in which a larger, aggressive mouse is allowed to bully a smaller mouse for a few minutes per day. During the rest of the time, the two mice are housed in the same cage but separated by a barrier, so they can still smell and see one another but cannot physically interact. Mice exposed to these conditions for either 10 days or 30 days showed changes in behavior believed to be signs of anxiety, such as spending less time in the center of an open area, which would be where a prey animal like a mouse would be most vulnerable to being picked off by a hungry predator.
Peering into the brains of these chronically stressed mice, Dr. Li’s team observed that animals that experienced 30 days of chronic social defeat had smaller mitochondria in the amygdala, a brain structure involved in processing emotions, particularly fear and anxiety. Mitochondria in the amygdala of those mice also produced significantly less energy than those of mice exposed to chronic social defeat for only 10 days or not at all. On the other hand, these mitochondrial changes were not seen in the hippocampus, a brain region involved in memory and the body’s stress response.
“Mitochondria are very sensitive structures,” Dr. Li explains. “They are normally like little sausages, but they can sense cellular stress, and when they sense this stress, they can undergo changes. They become smaller and rounder, like they are swollen.”
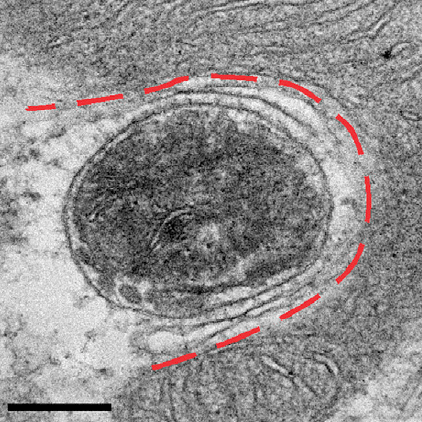
This electron microscope image from the study shows a mitophagosome, a cellular structure that breaks down dysfunctional mitochondria.
In addition, the researchers found that neurons in the amygdala contained fewer mitochondria in mice exposed to 30 days of chronic social defeat. The reason for this, it turned out, was that the animals’ brains revved up the removal of mitochondria from amygdala neurons via a process called mitophagy. Neurons in those mice had more structures, called mitophagosomes, that contribute to mitophagy by breaking down damaged mitochondria. Meanwhile, inactivating two genes known to be critical for mitophagy prevented chronic social defeat from increasing anxiety-like behaviors or reducing mitochondrial energy production in the amygdala. What’s more, directly damaging mitochondria specifically in the amygdala in mice that had not experienced social defeat increased both the number of mitophagosomes in amygdala neurons and the animals’ anxiety-like behaviors, establishing a direct causal link between mitophagy and anxiety.
“There is a very rigorous quality control mechanism for mitochondria in the cell,” Dr. Li says. “After social stress, when mitochondria show these unhealthy signs, the cell will sense these mitochondria need to be removed and will upregulate the mitophagy machinery to try to eliminate the bad mitochondria. And because the chronic social stress happens every day and is so intense, and the mitophagy is activating to such a high level, the mitochondria elimination is excessive — it’s faster than the creation of new mitochondria. The balance is broken, so there will be a net loss of mitochondria. With a loss of mitochondria, of course, the cell will not be very happy because it reduces the power supply.”
Mice that experienced chronic social defeat and displayed elevated anxiety also had weaker neuronal communication from the amygdala to a part of the brain called the bed nucleus of the stria terminalis (BNST), which the amygdala sends signals to in order to curb anxiety. However, artificially stimulating that neuronal pathway drove anxiety-like behaviors down to typical levels. Moreover, the diminished neuronal transmission induced by social defeat did not occur in mice that lacked one of those two mitophagy-related genes, suggesting once again that increased mitophagy was responsible.

Dr. Zheng Li
Dr. Li’s study is the first to establish a causal link between excessive mitophagy and anxiety and suggests that boosting mitochondrial energy production or curtailing mitophagy might alleviate anxiety disorders in some patients. Of course, additional studies, particularly examining cells from human volunteers, will be needed to translate the findings into treatments. For her part, Dr. Li plans to explore how mitochondria differ in different parts of the brain and in different types of cells in order to identify more specific targets for future therapies.
“When coming up with a treatment for psychiatric conditions, specificity is very important,” Dr. Li says. “In order to come up with a targeted drug, we need to know more about this brain region specificity and cell type specificity. I think that will be key to move forward to get more effective treatments.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
References:
[1] Mitophagy in the basolateral amygdala mediates increased anxiety induced by aversive social experience. Duan K, Gu Q, Petralia RS, Wang Y, Pania D, Liu X, Lehmann ML, Zhu H, Zhu J, Li Z. Neuron. 2021 Sep 27;S0896-6273(21)00680-2. doi: 10.1016/j.neuron.2021.09.008.
Related Blog Posts
This page was last updated on Tuesday, January 30, 2024
