Friendly Virus Could Deliver Gene Therapy Under Immune System’s Radar
IRP Research Points to New Tool for Alleviating Genetic Disorders
New IRP research suggests that a unique, harmless virus could be a potent tool for delivering gene therapies without triggering an immune response that would render the treatment ineffective.
With all the dangerous viruses out there, from the seasonal flu to the novel coronavirus that causes COVID-19, people understandably want to make sure their immune systems are topped-up with disease-fighting antibodies that block viral invaders. However, when it comes to the viruses scientists are modifying to deliver gene therapy, having a robust immune response is actually an obstacle to getting healthy. In a new study, IRP researchers showed that most people’s immune systems don’t react to a particular harmless virus that can effectively deliver new genes to the liver and heart, making it a promising delivery vehicle for therapies designed to alleviate a life-threatening genetic condition.1
While he was training at Children’s Hospital of Philadelphia in the early 2000s, new technology gave future IRP senior investigator Charles Venditti, M.D., Ph.D., the ability to diagnose infants with a genetic condition called methylmalonic acidemia (MMA) within days of their birth. Unfortunately, diagnosing the disease very early in life did little to alter its consequences.
“I was involved with meeting families and their babies that had methylmalonic acidemia and were diagnosed in the first one or two weeks of life,” he recalls. “The families would ask, ‘What’s going to happen to my baby?’ As a practitioner of metabolic medicine, I knew that the outlook was predicted to be grim, with a high chance of death and disability. The conversations were difficult.”
Learn more about Dr. Venditti's research on methylmalonic acidemia (MMA) in this video.
After that fellowship ended, Dr. Venditti came to NIH and began trying to come up with a way to correct the various genetic changes that can lead to MMA, thereby precluding the need for the restricted dietary regimens and liver transplants currently used to treat it. Enter fellow IRP senior investigator Jay Chiorini, Ph.D., an expert on using a harmless family of viruses called adeno-associated viruses (AAVs) in gene therapies. Viruses are extremely talented at injecting their own genetic material into cells, so with a bit of tweaking, benign viruses like AAVs can be co-opted as a delivery vehicle, or ‘vector,’ to give a healthy version of a mutated gene to people with genetic diseases.
Unfortunately, our immune systems don’t distinguish between viruses that are trying to help us and those that make us sick. Either way, exposure to a virus can cause immune cells in the blood to produce antibodies that neutralize it, which would render any virus-based gene therapy ineffective.
Those antibodies target a specific part of viruses called the capsid, which is essentially the coating that surrounds the virus’ genetic material, including any gene that doctors might be using an AAV to give to a patient. In the new study, Dr. Chiorini and Dr. Venditti’s labs teamed up to investigate the likelihood that an immune response would neutralize a variety of AAV called AAV44.9, which has a unique capsid. Dr. Chiorini’s lab discovered AAV44.9 a few years ago, and the researchers hoped its capsid would allow it to both avoid detection by the immune system and effectively carry genes into the right cells needed to treat MMA and other genetic disorders that cause similar problems.
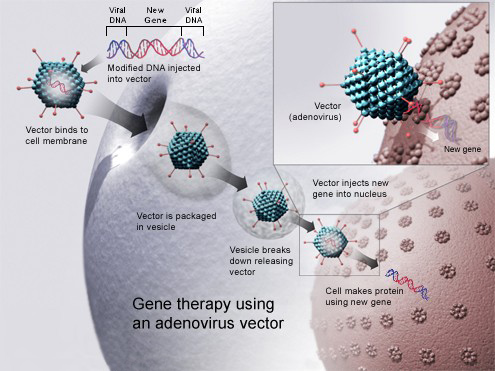
This diagram shows how a virus can be used to deliver a healthy copy of a dysfunctional gene to the cells of patients with genetic diseases.
“The capsid is the molecular truck of the gene therapy vector,” Dr. Chiorini says. “It dictates where the gene is going to be delivered and when it is going to be active, so it’s a really critical component of gene transfer technology.”
The IRP researchers tested blood samples from 19 healthy adults and 48 children with MMA to see if they had antibodies against AAV44.9. They discovered that only 26 percent of the adults and 13 percent of the children had the antibodies, and those who did had amounts of them that were likely too low to neutralize the virus.
“The low antibody reactivity to AAV44.9 was a very surprising finding,” Dr. Venditti says. “Most of our patients did not have antibodies against this capsid, meaning if we someday wanted to develop a gene therapy for our patients, this would be a very good candidate.”

Dr. Charles Venditti
Dr. Venditti was also heartened to see the very low level of pre-existing antibodies against AAV44.9 in healthy adults.
“Many of the vectors that researchers want to use in the clinic show high levels of pre-existing immunity in adults,” he explains. “The practical implication is that if you’re an adult and someone develops an AAV-based gene therapy, you might not be a candidate to receive it because of neutralizing antibodies, but if the therapy used AAV44.9 as the vector, our results suggest wider eligibility for the treatment over a large range of ages.”
When the researchers used AAV44.9 to deliver a gene to the cells of healthy mice that would make their cells emit light, they found that AAV44.9 effectively delivered the gene to the animals’ heart and liver cells. Moreover, an AAV44.9-based gene therapy designed to correct a mutation that causes MMA-like symptoms in mice proved extremely promising. Mice with a severe form of MMA that would typically live less than three days lived much longer when they received the gene therapy, and animals with a milder form of the illness lost less weight over time when given the gene therapy compared to untreated mice, a sign of improved health.

Dr. Jay Chiorini
While the results of the recent study suggest that AAV44.9 could be a highly effective way to deliver gene therapy for MMA, more work needs to be done before that can happen. Dr. Venditti is currently planning a clinical trial testing a gene therapy for MMA that uses a different viral vector his lab has been studying for longer than AAV44.9, but he hopes to test AAV44.9 for other genetic conditions similar to MMA in the future. What’s more, because so few patients with MMA had antibodies against AAV44.9 in the study, the virus could serve as a backup vector for gene therapy if patients have antibodies that neutralize other AAVs being used to deliver the treatments.
“Our collective goal is to widely enable gene therapies for patients with MMA and related disorders because we hope they will be ‘one and done’ treatments,” Dr. Venditti says. “Any new class of vector could help, especially ones that are very potent in living organisms, like AAV44.9”.
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
References:
[1] Chandler RJ, Di Pasquale G, Sloan JL, McCoy S, Hubbard BT, Kilts TM, Manoli I, Chiorini JA, Venditti CP. Systemic gene therapy for methylmalonic acidemia using the novel adeno-associated viral vector 44.9. Mol Ther Methods Clin Dev. 2022 Sep 6;27:61-72. doi: 10.1016/j.omtm.2022.09.001. eCollection 2022 Dec 8.
Related Blog Posts
This page was last updated on Tuesday, May 23, 2023
