Unlocking the Genetic Mysteries of Rare Autoinflammatory Diseases
IRP Researcher Finds Explanations and Hope
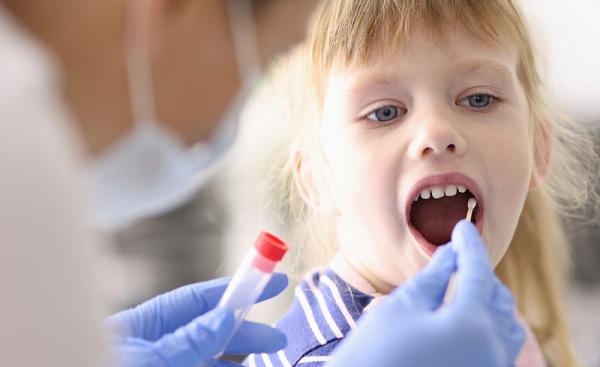
Sampling the DNA of children with rare inflammatory conditions has allowed IRP researchers to find treatments for several of the mysterious ailments.
Rare Disease Day, celebrated on or near February 29 — the rarest day on the calendar — calls attention to the 300 million people in the world who have some sort of rare disease. For children born with one of those diseases, speedy diagnosis and treatment may be necessary to ward off long-term complications, but that’s much easier said than done. This is especially true for pediatric autoinflammatory diseases, in which the immune system attacks the child’s own body. IRP senior investigator Raphaela T. Goldbach-Mansky, M.D., M.H.S., has made it her mission to discover and define these diseases and the genes that cause them, and then find a way to provide treatment.
“Our research becomes care for these patients because otherwise we wouldn’t have good treatment options.” Dr. Goldbach-Mansky says. “The need to treat these little kids and make them better has dictated the structure of my research program.”
The diseases Dr. Goldbach-Mansky studies are triggered by molecules that are meant to sense dangers like infections or tumors and begin mounting an immune defense against them. However, the children she sees develop unexplained rashes, fevers, pain, and inflammation — all effects of an immune response — but without any true threat to set it off.

The children Dr. Goldbach-Mansky’s team treats experience rashes, fevers, and inflammation that have nothing to do with any kind of infection or other threat that would normally trigger such symptoms.
Dr. Goldbach-Mansky came to NIH as a fellow in 1997, where she met IRP Distinguished Investigator Dan Kastner, M.D., Ph.D., who had found the first gene responsible for a condition called familial Mediterranean fever. This discovery made it clear that a single genetic change could cause a complex inflammatory disease. Dr. Goldbach-Mansky took that idea and applied it to the unclassified inflammatory diseases she saw in the children she treated.
“We’ve always hypothesized that these sporadic, severe diseases might be caused by new mutations,” Dr. Goldbach-Mansky says. Over the years, she and her team have discovered 11 autoinflammatory diseases and 18 disease-causing genes. Their discoveries have led to actual treatments for several of the diseases they’ve found.
Two of those diseases, called NOMID and DIRA, are caused by mutations that disrupt an immune protein called interleukin-1 (IL-1), which in normal circumstances helps the body fight infections and inflammation. Once Dr. Goldbach-Mansky and her laboratory pinpointed the genes responsible, they were able to deduce what was going wrong with IL-1 in patients with those conditions and block its overproduction with a drug called anakinra.
“We conducted a proof-of-concept study1 in 2003 that showed improvement within days of treatment,” says Dr. Goldbach-Manksy. “These children who had never been rash-free or who screamed every night from pain were finally able to sleep through the night. The rashes disappeared and the headaches went away. It was a great success.” Anakinra has since been approved for use in treating NOMID and DIRA.
While it wasn’t possible to sequence her patients’ DNA when Dr. Goldbach-Mansky was searching for answers in children with NOMID and DIRA, her laboratory gained the ability to do so for other conditions as the cost of sequencing fell throughout the first decade of the new millennium. For instance, in 2012 her team was able to do ‘trio analysis,’ in which she sequenced the DNA of the child and both parents and then looked for mutations that the parents didn’t have.2 This method helped her team find the mutation responsible for a disease caused by overproduction of another immune system chemical called interferon type 1.

Dr. Raphaela Goldbach-Mansky
Armed with this knowledge, Dr. Goldbach-Mansky's team designed a clinical trial3 that tested whether a drug called baricitnib, which blocks interferon signaling and was already available for rheumatoid arthritis, could help patients with one of two rare conditions called CANDLE and SAVI.
“In CANDLE, we saw that 50 percent of patients treated with baricitinib went into complete remission,” Dr. Goldbach-Mansky says. “We also had responses in SAVI, but not to the extent of actual remission.”
Then, about three years ago, another drug her team had investigated became available when it was approved as a treatment for lupus. This drug, anifrolumab, is now being tested in children with SAVI and CANDLE.
“We can completely suppress the interferon signature and have made huge strides in patients with SAVI before they actually develop severe lung diseases,” Dr. Goldbach-Mansky says. “The drug also does a good job at preventing the chronic skin ulcers that are common with this disease.”
This sort of research fills in an important gap in traditional, private-sector research. Because the number of patients with each rare disease is so small, drug manufacturers have little economic incentive to put in the money and time to develop new treatments for them. However, by identifying the cause of a rare disease, researchers like Dr. Goldbach-Mansky can see if patients respond to existing drugs that haven’t been approved for their condition. The trick is to figure out which one works, and then — even more difficult — find enough patients to test it on so that any positive results can’t be dismissed as a fluke.
“Currently, we don’t have mechanisms to get FDA approval for a drug to treat a disease only 5 to 10 people have,” Dr. Goldbach-Mansky says. “There’s no way to have traditionally phased clinical studies because we just don’t have enough patients.”
To address the critical unmet need, Dr. Goldbach-Mansky founded the Translational Autoinflammatory Diseases Network (TARN) and is working with others to develop and refine new clinical trial models that can merge the traditional phases of drug development — from determining the dose to safety follow-up — into a single clinical study with the same patients. Her team is also working with the FDA to define disease reporting terms and standardize data collection from these trials in such a way that their findings could be used for drug approvals.
“These changes could be very important for moving the field forward, as well as allowing secure access to treatment for the patients,” Dr. Goldbach-Mansky says. “And often, when a drug gets approved in the U.S., it also gets approved in other countries, so our work may have far-reaching consequences beyond the borders of the U.S.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
References:
[1] Goldbach-Mansky R et al. Neonatal-onset multisystem inflammatory disease responsive to interleukin-1beta inhibition. N. Engl. J. Med. 2006 Aug. 10; 355(6):581-592. doi: 10.1056/NEJMoa055137.
[2] Liu Y, et al. Activated STING in a vascular and pulmonary syndrome. N Engl J Med. 2014 Aug. 7; 371:507-518. doi: 10.1056/NEJMoa1312625.
[3] Sanchez GAM, et al. JAK1/2 inhibition with baricitinib in the treatment of autoinflammatory interferonopathies. J Clin Invest. 2018 Jul 2;128(7):3041-3052. doi: 10.1172/JCI98814.
Related Blog Posts
This page was last updated on Friday, February 28, 2025
