HHS Awards Recognize IRP Cancer Researchers
Long Careers at NIH Yield Groundbreaking Achievements
When you work at the National Institutes of Health, major advances in health and science can seem like a regular occurrence. Yet not all advances are created equal; some change entire paradigms for understanding and treating disease, even disarming a disease’s lethal effects.
This fall, three IRP senior investigators received Departmental Awards from the United States Department of Health and Human Services (HHS) for their exceptional contributions to science: Louis M. Staudt, M.D., Ph.D., Elaine S. Jaffe, M.D., and Robert Yarchoan, M.D. Dr. Staudt received the HHS Secretary’s Award for Distinguished Service for his revolutionary work on the diagnosis and treatment of diffuse large B-cell lymphoma (DLBCL); Dr. Jaffe received the Secretary’s Award for Meritorious Service for her pioneering discoveries about lymphomas and blood cancers; and Dr. Yarchoan received the HHS Career Achievement Award for his role in developing the first effective drugs for AIDS and developing treatments for HIV-associated cancers.
Read on to learn more about Dr. Jaffe’s and Dr. Yarchoan’s groundbreaking research, and when you’re done, listen to Dr. Staudt himself describe his life-saving work on the IRP’s Speaking of Science podcast.
If You Can Name It, You Can Tame It

Dr. Elaine Jaffe received the HHS Secretary’s Award for Meritorious Service in recognition of her contributions to the improved diagnosis and treatment of lymphomas and other cancers affecting the blood.
In 1832, Thomas Hodgkin, anatomist and “Inspector of the Dead” at Guy’s Hospital in London, described enlarged lymph nodes in seven patients. Hodgkin’s disease, as the condition was later named, turned out to be one of many forms of cancer that can afflict the lymph nodes. However, for nearly two centuries, it was the only one to get its own name. The rest were lumped together as non-Hodgkin’s lymphoma — essentially a medical term for ‘everything else.’ Yet these other lymphomas represent a huge variety of tumors affecting the lymphatic system, each with its own molecular characteristics and genetics.
“Back in the early 1970s, we were really just beginning to learn about lymphoma and lymphoid tumors,” Dr. Jaffe recalls of the beginning of her medical career. “We recognized only a handful of lymphoma types. It was as basic as ‘was it Hodgkin’s or non-Hodgkin’s disease.' Non-Hodgkin’s, which we now understand is made up of more than a hundred different cancers, was lumped into one big black box.”
For Dr. Jaffe, the key to unlocking this black box was her microscope. As a second-year medical student, she had found her calling in her pathology class.
“I really saw the field of medicine come alive under the microscope — that was where I could see the nature of the disease,” Dr. Jaffe says. “It drove my interest in trying to understand disease and better diagnose it because through accurate diagnosis we achieve better therapy.”
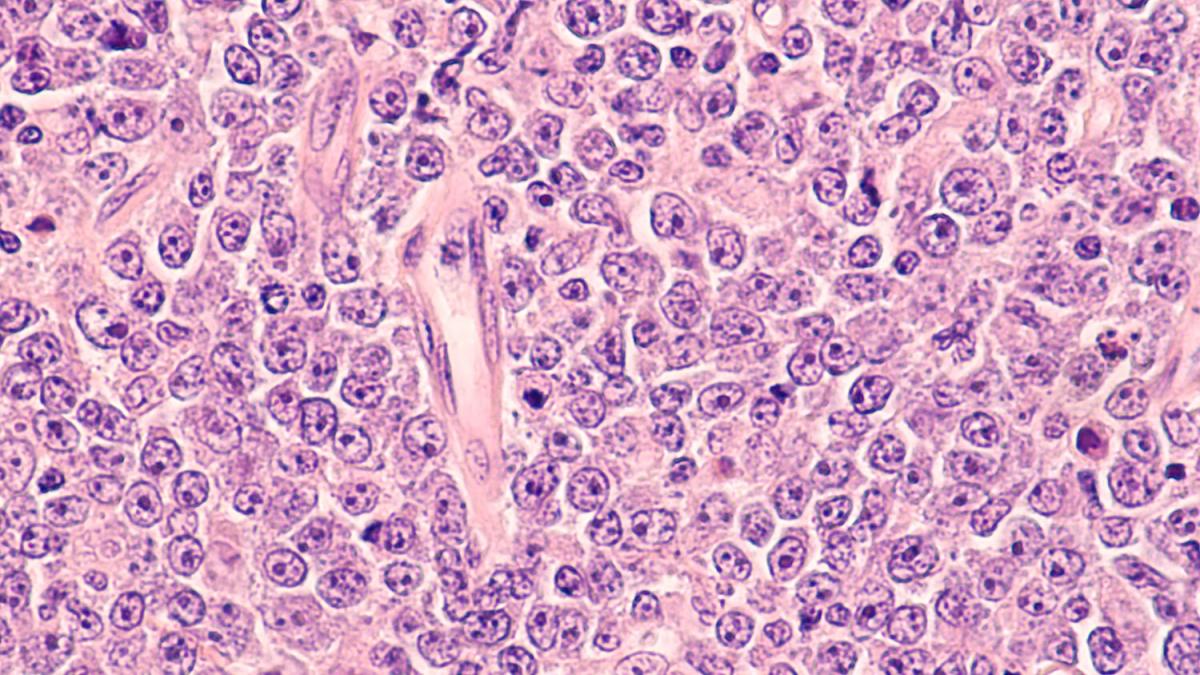
When scientists like Dr. Jaffe peer into their high-powered microscopes, they might see something like this image of diffuse large B cell lymphoma, a type of non-Hodgkin's lymphoma.
A year after completing a year-long pathology residency at Georgetown University, a relative encouraged Dr. Jaffe to come to the Laboratory of Pathology at NIH’s National Cancer Institute (NCI) in 1970. It was the right place at the right time.
In the early 1970s, two NCI scientists, Paul Carbone, M.D., and Vincent DeVita, M.D., had shown that chemotherapy could cure some patients with Hodgkin’s disease and certain other forms of lymphoma. At the same time, immunologists were developing the tools and techniques needed to understand the human immune system, and with that, recognize and distinguish between different types of immune cells, including those produced within the lymphatic system. Those cells, called lymphocytes, include B cells, which produce antibodies that mark specific pathogens for destruction, and T cells, which identify foreign invaders and destroy them directly. When something in these cells goes awry, they become caricatures of themselves, Dr. Jaffe says. While they retain features and functions of normal immune cells, they begin the excessive replication characteristic of cancerous cells and form tumors.
“It was a time when the different types of lymphocytes were first being identified, but we didn't know how this related to the different types of lymphoma,” Dr. Jaffe says. “I was using very primitive tools available at the time to identify T cells and B cells under the microscope to determine the type of lymphoma we were dealing with.”
Early in this process, Dr. Jaffe studied tumors called nodular lymphomas. Now called follicular lymphomas, these tumor cells form nodules — small lumps — that can be seen under a microscope , but at the time no one really understood their significance. Working with her mentor, Costan W. Berard, M.D., and colleagues across the IRP, including Ethan M. Shevach, M.D., Dr. Jaffe was able to show the nodules were there because the affected cells were derived from normal lymphoid follicles, a type of bodily tissue where B cells encounter and react to cells or substances and produce bespoke antibodies able to stick to them.
“We showed that what we were calling nodular lymphoma was actually a tumor of follicular B lymphocytes.,” Dr. Jaffe says. “That was the beginning of the revolution of relating lymphomas to the normal immune system.” The 1974 paper describing these findings was so fundamental to the field, it ultimately became a 'citation classic.'1
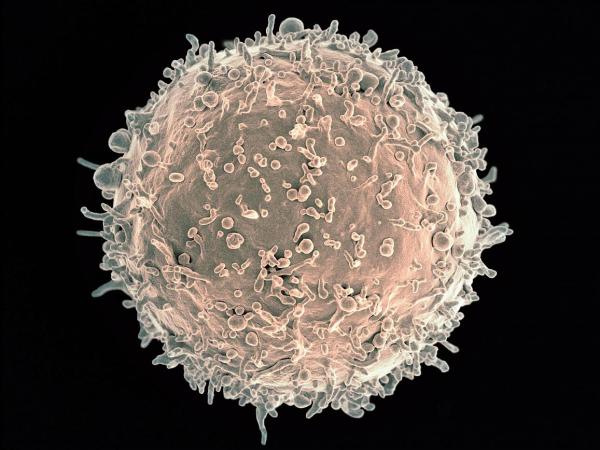
Dr. Jaffe helped discover that so-called ‘nodular lymphoma’ emerged due to cancer-inducing changes to immune cells called B cells, pictured here.
Nowadays, the medical field recognizes at least 100 different types of lymphoma, and Dr. Jaffe has spent a significant part of her career helping to distinguish, characterize, and understand them so that we can better treat them.
“The treatment of lymphoma has become much more complex and more targeted,” she says. "Knowing exactly what the tumor is — what is driving the individual tumor cells — is very important in therapy.”
Classifying these different lymphomas is critical to improving therapies, but different systems for categorizing them and naming them cropped up across different regions and even between researchers and clinicians. To bring order and continuity to the field, Dr. Jaffe joined with others in her field around the world to create the International Lymphoma Study Group in 1991. After working together for three years, the group published the Revised European-American Lymphoma (REAL) classification system.2
Today, Dr. Jaffe remains a prominent figure in her field. While she continues her research into the microscopic characteristics — and now genetics — of the cells that make up tumors of the immune system, the many trainees that she has mentored have carried her expertise and appreciation for the intricate microscopic markers of disease across the world.
“I have fellows at just about every major medical center around the country, and it's very gratifying to see them succeed and follow in my footsteps,” Dr. Jaffe says. “We refer to them as the apples because the apples don't fall far from the tree. I think of all my fellows as my apples.”
A Ray of Light During AIDS’ Darkest Hour

Dr. Robert Yarchoan received the HHS Career Achievement Award for his role in developing the first effective drugs for AIDS, as well as treatments for HIV-associated cancers.
Like Dr. Jaffe, Dr. Yarchoan found himself at the center of a major medical upheaval early in his career. In his case, he was finishing up an immunology fellowship in NCI’s Metabolism Branch, led at the time by Thomas A. Waldmann, M.D. As part of their research on the human immune system and related diseases, the two physician-scientists often treated patients at the NIH Clinical Center with illnesses that weaken the immune system or cancers that affect it.
Just as Dr. Yarchoan was determining his next career move, reports of a strange, deadly disease that devastated the immune system began cropping up in major U.S. cities. Dr. Yarchoan soon took his place on the front lines of the burgeoning AIDS crisis.
“We had a young gentleman from New York come in with a severely weakened immune system,” Dr. Yarchoan recalls. “A couple of weeks later, the Centers for Disease Control came out with reports on the first AIDS patients, and it became clear that this patient we were treating had this newly identified syndrome.”

The burgeoning AIDS crisis of the 1980s was the subject of both intense scientific inquiry and extensive media coverage.
In fact, as he later recalled in an oral history of NCI’s work during the early years of the AIDS crisis, this patient was a “textbook case of AIDS,” with a severe lack of disease-fighting T cells in his blood and multiple ‘opportunistic’ infections that only afflict people with weakened immune systems. It was later determined that AIDS kills off a particular type of T cell, rendering the immune system nonfunctional.
In the laboratory next door, Samuel Broder, M.D., had been tasked with setting up a small group to figure out how to treat this new disease. He invited Dr. Yarchoan, along with Hiraoki “Mitch” Mitsuya, M.D., Ph.D. — also a postdoctoral fellow at the time — to join him. The trio began searching for potential drugs that could work.
“I was in the right place at the right time and took advantage of the situation,” Dr. Yarchoan says. “The three of us made a great team with really complementary skills. Mitch was great in the lab, I had a good nose for running clinical trials, and Sam was very good at tapping into a lot of resources in the NCI and was a good team leader.”
In those early days, no one knew what was causing AIDS, so coming up with a treatment was a challenge. Fortunately, NCI’s Robert Gallo, M.D., had discovered the first human retrovirus, HTLV-1, a few years earlier. Since HLTV-1 seemed to weaken the immune system, Dr. Yarchoan and his two colleagues thought it might be a good model to study what was going on with AIDS. Once Dr. Gallo’s lab showed that the virus now called HIV was the actual culprit behind AIDS, the trio redirected their work and focused exclusively on finding a drug that would fight that virus.
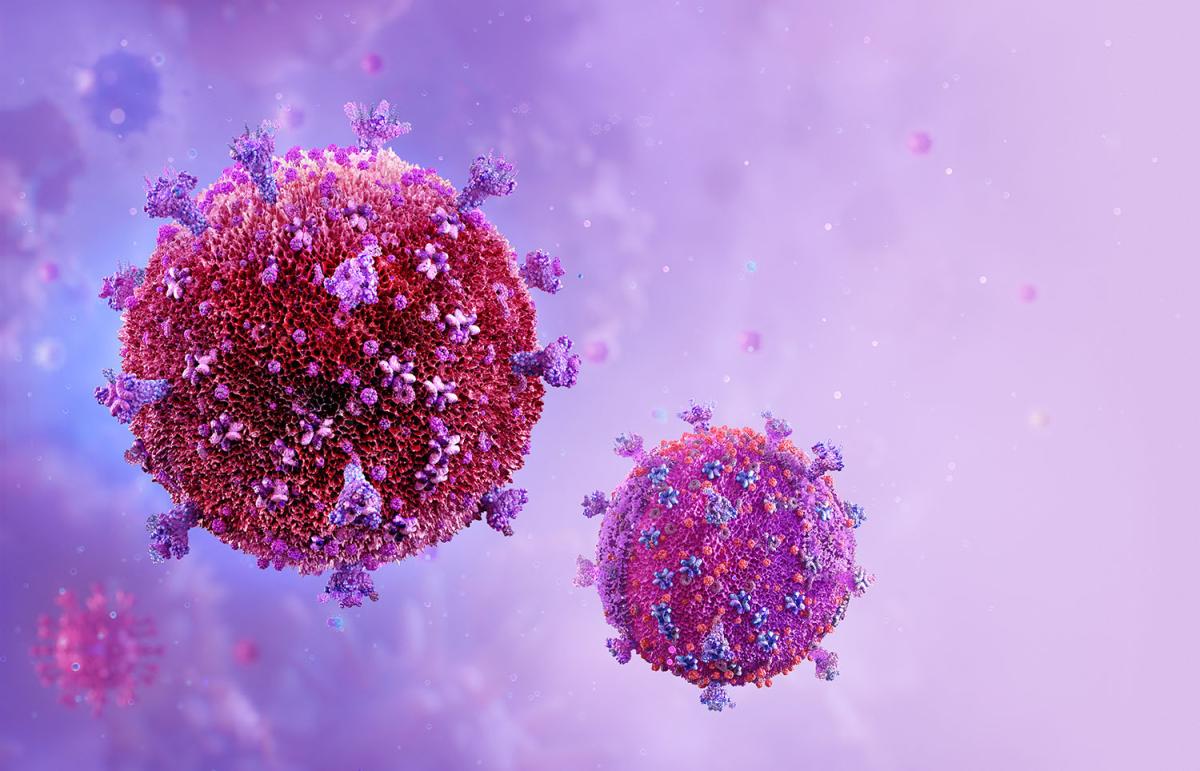
The discovery of the particular virus that causes AIDS, now known as HIV, was key to the development of drugs to combat the life-threatening condition.
That search wasn’t easy. Although they had some ideas for the type of drug that might work, finding it required developing a way to test it and then selecting a candidate drug to evaluate. An added challenge was figuring out how to gauge the drug’s effectiveness in a disease like AIDS in which most of the symptoms are caused by the other, opportunistic infections that invade the body once HIV has weakened the immune system. On top of that, even though it was clear an epidemic was taking place, AIDS was still considered a rare disease since it only affected about 12,000 people, so it was difficult to find a pharmaceutical company willing to help develop a drug for such a small market.
One company, Burroughs Wellcome Co., had expertise in nucleoside analogs, one of the types of compounds Drs. Mitsuya, Broder, and Yarchoan were interested in as potential AIDS therapeutics. The company agreed to collaborate with NCI on testing them, and the company’s collaboration with the NCI trio’s team found that one compound, azidothymidine (AZT), showed promise in laboratory tests. The scientists then showed that it had benefits in human patients in a small Phase I clinical trial conducted in the NIH Clinical Center. Ultimately, AZT became the first antiretroviral to be approved for HIV/AIDS and the first treatment to provide hope to thousands of affected patients.

AZT crystals viewed under a microscope using polarized light.
Although AZT was a true breakthrough, it eventually became evident that its effectiveness waned after about five months as resistance to the drug kicked in. To look for other treatments, Drs. Yarchoan, Broder, and Mitsuya began buying chemical compounds from manufacturers around the country, looking for formulations that do a better job stopping HIV. This time, they developed the drugs and tested them at NIH themselves. This resulted in two additional drugs, didanosine (ddI) and zalcitabine (ddC). Not only did each of these drugs work by itself, but Dr. Yarchoan and his colleagues also showed they worked even better when used together. Their discoveries revolutionized HIV/AIDS treatment,3 and later these drugs provided the backbone of the commonly used three-drug regimen known as highly active antiretroviral therapy (HAART).
Since that breakthrough, with the help of a variety of IRP collaborators, Dr. Yarchoan has continued to make important advances in treating some of the cancers and viral diseases associated with HIV/AIDS. He led clinical trials for paclitaxel, now sold under the brand name Taxol, which became one of the main treatments for Kaposi’s sarcoma, a cancer that occurs in people with weakened immune systems. His research team also showed that a drug called pomalidomide could help patients with Kaposi’s sarcoma as well, leading to its FDA approval for that purpose. In addition, he developed new treatments for two diseases caused by Kaposi’s sarcoma-associated herpesvirus (KSHV), multicentric Castleman’s disease and KSHV-Associated Cytokine Syndrome (KICS).
“We often give lip service to NIH’s Intramural Research Program being a unique environment, but in the work I'm doing, it really is unique,” Dr. Yarchoan says. “Given the fact that most people in the United States with HIV are getting good treatment, there are not that many cases of Kaposi’s sarcoma here.” However, its prevalence is increasing among gay, Black men in the rural South. “The fact that we can draw people from around the country to the NIH Clinical Center has enabled us to work with more of these patients compared with anywhere else in the country. As a result, our clinicians have become very good at treating them and have gained so much expertise.”
“It's been very gratifying to see the work that we do in our little lab and the Clinical Center having this major worldwide impact,” Dr. Yarchoan adds. “These drugs have really saved millions and millions of lives. They’ve been shown to block maternal-fetal transmission of HIV, so they've prevented children from being infected with HIV. It has really been humbling to see.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
References:
[1] Jaffe E, Shevach EM, Frank MM, Berard CW, Green I. (1974). Nodular lymphoma—Evidence for origin from follicular B lymphocytes. N Engl J Med 290:813–819. doi: 10.1056/NEJM197404112901501.
[2] Harris NL, Jaffe ES, Stein H, Banks PM, Chan JK, Cleary ML, Delsol G, De Wolf-Peeters C, Falini B, Gatter KC. (1994). A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood 84:1361–1392. doi:10.1182/blood.V84.5.1261.1361.
[3] Yarchoan R, Mitsuya H, Thomas RV, Pluda JM, Hartman NR, Perno CF, Marczyk KS, Allain JP, Johns DG, Broder S. (1989). In vivo activity against HIV and favorable toxicity profile of 2',3'-dideoxyinosine. Science. 245(4916), 412-5. doi: 10.1126/science.2503840.
Related Blog Posts
This page was last updated on Wednesday, January 3, 2024
