Jekyll-and-Hyde Gene Has Dual Influences on Dementia Risk
IRP’s Priyanka Narayan Explores How the ApoE Gene Affects Fat Management in the Brain

IRP researchers are exploring how different versions of a single gene can either raise or lower the risk for Alzheimer’s disease and other forms of dementia.
Just as Dr. Jekyll and Mr. Hyde exhibited the extremes of good and bad qualities in a single man, IRP Stadtman Investigator Priyanka Narayan, Ph.D.,is showing how a single gene can both protect against and raise the risk for Alzheimer’s disease and other dementias. As we observe Alzheimer’s and Brain Awareness month this June, we talked with Dr. Narayan about her work.
“Our research focuses on understanding fundamental cell biology in brain cells and how genetic factors affecting that biology can predispose individuals to Alzheimer's disease,” Dr. Narayan says. “More recently, we’ve become interested in protective factors that make people more resistant to getting Alzheimer’s disease and other forms of neurodegenerative diseases as well.”
Alzheimer’s disease is the most common form of dementia, affecting nearly 7 million people in the U.S. Troublingly, that number is expected to double to 14 million by 2060. The condition’s cause is not fully understood, and it is likely that numerous factors influence a person’s risk for it, from genes and age-related biological changes to environmental exposures and lifestyle factors.
Dr. Narayan is taking a fresh look at that question by examining fat storage and inflammation in brain cells. Rather than looking at the amyloid-beta ‘plaques’ and tau ‘tangles’ that most Alzheimer’s researchers have long focused on, she and her team are studying the role of fat molecules called lipids, as well as the transporters that carry them in the brain.
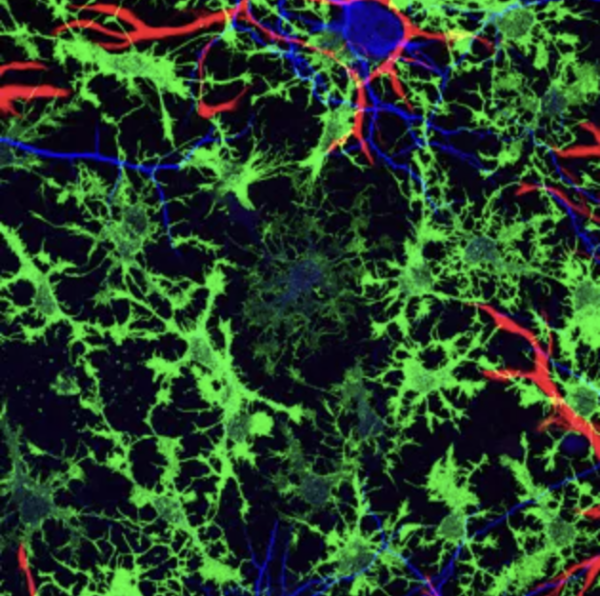
Neurons (blue) rely on multiple types of supportive glial cells (red and green) in order to function properly, but most Alzheimer’s research has not focused on this second sort of brain cell.
Specifically, her team is looking at how lipids are managed in a category of cells called glia, which have been traditionally thought of as support cells for communication between neurons. However, it is becoming clear that lipids also play a role in the development and progression of degenerative brain diseases.1 In fact, one of the strongest risk factors for neurodegenerative disease is a specific variant of a gene that codes for a lipid transport protein, called ApoE. That genetic variant, known as ApoE4, drives one of the most common forms of Alzheimer's disease, and Dr. Narayan’s experiments have revealed that it causes glial cells involved in the brain’s immune response, called microglia, to hoard lipids.
“What we have found in our studies is that lipids are actually necessary for the inflammatory response in microglial cells,” Dr. Narayan says, “but when the ApoE4 gene is present, these lipid production pathways are hijacked, causing chronic inflammation.”2
“We've realized that the ApoE4 gene variant causes lipid storage defects,” Dr. Narayan continues. “You have these large lipid droplets in the glial cells that you don’t see in cells with the ApoE3 variant, which is the healthy, benign version of the gene with respect to disease.”
Now, Dr. Naryan’s lab is trying to learn what causes that hoarding in the first place and how it impacts disease risk.
“We hope we can manipulate the hoarding to reduce disease,” she says.

Dr. Priyanka Narayan
On the protective side, Dr. Narayan and her team are looking at other variants of the ApoE gene that are associated with a lower risk of Alzheimer’s disease.
“We've known ApoE2 is a protective variant for a long time, but recently there have been other protective variants that have emerged from epidemiological studies,” Dr. Narayan says. “This has been really exciting because now we know that there might not be just one way to protect against Alzheimer’s.”
To pursue that possibility, Dr. Narayan’s team is trying to figure out whether these protective variants of the ApoE gene are better at regulating lipid storage and inflammation compared with the neutral ApoE3 variant and the harmful ApoE4 variant, or if something else is going on. One thing they have discovered is that genetic contributions to Alzheimer’s risk lie on a spectrum, rather than being binary. For one thing, we each inherit two copies of the gene, so different combinations of genetic variants can affect the levels of risk and protection. Other factors related to the ApoE gene may also play a role, so Dr. Narayan’s team is trying to determine the specific mechanisms by which each gene variant influences susceptibility to, or protection from, neurodegeneration.
“In terms of science, I think these protective gene variants are our biggest new frontier,” Dr. Narayan says, “but it will take us a while to sort through that.”
“A lot of people are working on ApoE; it’s not an empty field,” she adds, “but this is what we can contribute uniquely to the larger field that sets us apart, even if we're a small team. And I think that it will be a fun journey to start figuring it out.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
References
[1] Yang LG, March ZM, Stephenson RA, Narayan PS. Apolipoprotein E in lipid metabolism and neurodegenerative disease. Trends Endocrinol Metab. 2023;34(8):430-445. doi: 10.1016/j.tem.2023.05.002.
[2] Stephenson RA, Johnson, KR, Cheng L, Yang, LG, Root, JT, Gopalakrishnan J, Shih H-Y, and Narayan PS (2024). Triglyceride metabolism controls inflammation and APOE4-associated disease states in microglia. bioRxiv, 2024.04.11.589145. doi: 10.1101/2024.04.11.589145.
Related Blog Posts
This page was last updated on Thursday, June 13, 2024
