The SIG Beat: Redox Biology SIG
News From and About the Scientific Interest Groups
Redox Biology SIG: A Reactive History of Redox Research at NIH
The duality of oxygen was beginning to reveal itself in the 1970s when some scientists were drawing attention to the oxygen paradox—a contentious concept that while oxygen is essential for survival of all aerobic life forms, it can also elicit toxicities through the generation of reactive species known as free radicals.
“As a neonatologist, we knew all about oxygen toxicity,” said Senior Investigator Rodney Levine at the National Heart, Lung, and Blood Institute (NHLBI). In the 1970s Levine had been treating premature infants whose lungs had been damaged from being ventilated with too much oxygen. (Excess oxygen could also cause blindness in these babies.) Levine’s experience was but one vignette in the nascent field of redox biology that would be enriched by a passionate group of investigators who hailed from a range of scientific disciplines.
When they are breathing, all aerobic organisms produce reactive molecules through redox reactions: chemical reactions in which the oxidation number of a molecule, atom, or ion changes by gaining or losing an electron. This normal electron swapping plays a vital role in maintaining functions such as cellular metabolism, molecular signaling, immune response, and the removal of toxins. But those same redox reactions can also be harmful during oxidative stress, a condition of imbalance between free radicals and antioxidant defenses (consisting of small molecules that sacrifice an electron to neutralize free radicals and enzyme systems that degrade reactive species) in the body. Chronic oxidative stress may lead to cardiovascular diseases, cancer, neurodegeneration, and even complications in preterm newborns.
As data surrounding oxygen toxicity accumulated “it was becoming necessary to bring people together to understand the chemistry and biochemistry of it,” said Carol Colton, who is a neuroscientist at Duke University (Durham, North Carolina) and was a special expert (1985-1987) in Daniel Gilbert’s Reactive Oxygen Species Unit lab at the National Institute of Neurological Disorders and Stroke. In 1987 the pair co-founded The Oxygen Club of Greater Washington, D.C. and its sister NIH Free Radical Research Scientific Interest Group (SIG).
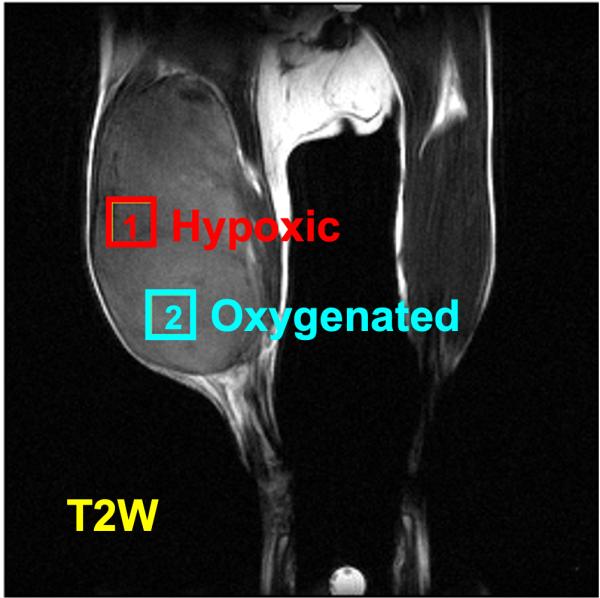
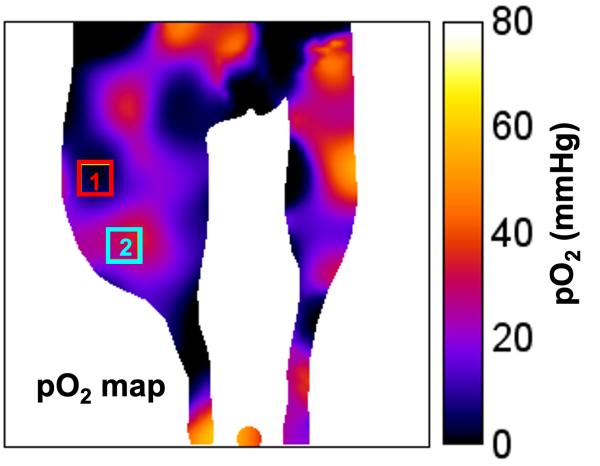
CREDIT: S. MATSUMOTO ET. AL., J CLIN INVEST
NCI and NINDS investigators used a mapping technique to show oxygen gradients in tumors. Left: An MRI of a tumor in a mouse leg. Right: a pulsed electron paramagnetic resonance image of the same tumor showing oxygenated (2) and hypoxic (1) regions (J Clin Invest 118:1965–1973, 2008).
Colton considers Gilbert “the grandfather of the movement.” In the mid-1950s, Gilbert and Rebeca Gerschman were the first to recognize a common free radical mechanism of action in oxygen toxicity and radiation (Science 119:623-626, 1954). Gilbert, Colton, and their colleagues would go on to generate seminal work on the role of oxygen in the brain, which led the way in understanding how seizures may come about (Adv Neurol 59:321-326, 1993). The new SIG was among the first transdisciplinary scientific groups dedicated to understanding the role of oxygen, free radicals, reactive oxygen, and nitrogen species across the different fields of biology, physiology, and medicine. It provided investigators the opportunity to learn and network with colleagues at NIH and across the country by organizing lectures, seminars, and meetings.
“These meetings gave [us] the ability to talk to people who are dealing with chemistry, with clinical applications, with basic biology and applied biology,” said William F. Blakely, an early member of the original Oxygen Club and now a senior staff scientist at the Armed Forces Radiobiology Research Institute (Bethesda, Maryland).
In 1977, Levine joined Earl Stadtman’s Laboratory of Biochemistry at NHLBI as a postdoctoral fellow to work on protein turnover. Stadtman was an international redox leader himself and had made major contributions toward understanding the duality of oxygen. His lab both uncovered how oxidation can degrade proteins, and as early as 1960, showed how a free radical played an essential role in cellular metabolism (J Am Chem Soc 82:2643-2644, 1960). In 1987, Levine and Stadtman helped found a national society now known as the Society for Redox Biology and Medicine. A wave of new findings in the field led to a lot of excitement and the formation of other oxygen clubs across the country, which began to collaborate. “It was all happening in continuum,” said Levine.
Sue Goo Rhee, currently a special volunteer at NHLBI, is yet another redox pioneer who began his postdoctoral training at Stadtman’s lab in 1973 (Antioxid Redox Signal 34:1-10, 2021). Rhee was instrumental in identifying hydrogen peroxide as a crucial signaling molecule within cells and discovered peroxiredoxins—a class of antioxidants that regulate hydrogen peroxide concentrations (Sci Signal 2000; DOI:10.1126/stke.2000.53.pe1, 2000). Hydrogen peroxide is a major redox metabolite: Left unchecked it can be toxic, but when controlled it modulates redox-sensitive proteins involved in pathways that facilitate cell differentiation, proliferation, metabolism, survival, and immune response.

CREDIT: JOHNS HOPKINS SCHOOL OF MEDICINE
NCI’S Stadtman Tenure Track Investigator Urbain Weyemi chairs the rebranded Redox Biology SIG.
In 2022, the Free Radical Research SIG was rebranded as the NIH Redox Biology SIG, chaired by National Cancer Institute’s (NCI) Stadtman Tenure Track Investigator Urbain Weyemi. Weyemi had been studying how reactive oxygen species play a role in neurodegenerative diseases and is especially interested in harnessing the dialogue between redox metabolism and genome repair in new ways to treat cancer. He hopes to revitalize the original spirit of the club in the modern era along with the mentorship of original SIG members Levine and Michael Espey, chief of NCI’s Radiotherapy Development Branch. “This virtual format evolution that we are currently experiencing literally didn’t exist back then,” said Espey, recalling the days when communication relied on word of mouth, handbills, and NIH’s yellow sheet, which would announce seminars.
The NIH Redox Biology SIG held its inaugural talk in September 2022, with Boyi Gan, professor in the Department of Experimental Radiation Oncology, MD Anderson Cancer Center (Houston). Gan has made groundbreaking discoveries on the role of ferroptosis in cancer. Discovered just 10 years ago, ferroptosis is a form of cell death characterized by iron accumulation and oxidative damage to cell membranes. The seminar was attended virtually by almost 100 people across the country and beyond.
“We have already gathered 200 subscribers [to our new LISTSERV email newsletter] in just two months,” said Weyemi, who is inviting a senior investigator or a “big star” of redox biology each month for the seminar series. Starting in September 2023, the SIG will host a new format of short talks by young investigators or “rising stars” in the field.
The SIG’s leaders hope that redox biology can be an engine to drive better science. For example, scientists typically culture cells for laboratory experiments at room air conditions, or 21% oxygen. However, inside most organisms, cells are exposed to much lower levels of oxygen. Considering that fact when designing experiments could lead to more biologically meaningful data.
“As the years go by, new discoveries [in redox biology] peel back layers of the onion,” said Espey, highlighting the importance of collaborating with colleagues and synthesizing their findings to build knowledge. “I feel that is where the real power is.”
For more information about the Redox Biology SIG including instructions for joining the LISTSERV newsletter, go to https://oir.nih.gov/sigs/redox-biology-interest-group. All meetings will be held virtually for the foreseeable future. Intramural researchers as well as those outside of NIH are welcome to join the LISTSERV and receive links to upcoming seminars. For more information contact Urbain Weyemi at urbain.weyemi@nih.gov.
Satabdi Nandi, a postdoctoral fellow in the Laboratory of Molecular Biology and Immunology in the National Institute on Aging, is investigating the generation of antibody diversity in mouse B cells. Outside of work, she enjoys visiting museums to learn about the past, diverse cultures, and the motivation behind artists’ creations.
This page was last updated on Wednesday, November 9, 2022
