An Old Virus Gets New Attention
Worldwide Outbreak Puts a Fresh Focus on Monkeypox Research
For decades, monkeypox has been exclusively endemic to parts of Central and West Africa. But in 2003, 3-year-old Schyan Kautzer of Dorchester, Wisconsin, developed flu-like symptoms and a painful rash of raised welts after being bitten by her pet prairie dog. She was the first person diagnosed with monkeypox in the Western Hemisphere, followed by more than 70 suspected or confirmed cases in six midwestern states reported to the Centers for Disease Control and Prevention (CDC). And the likely culprits? Infected Gambian pouched rats imported from Ghana. The rodents were housed next to a shipment of prairie dogs that in turn became infected before being distributed through the exotic pet trade. All confirmed cases of monkeypox in humans were eventually traced to direct contact with those prairie dogs. Within two months the outbreak would fizzle out, along with international attention on the virus.
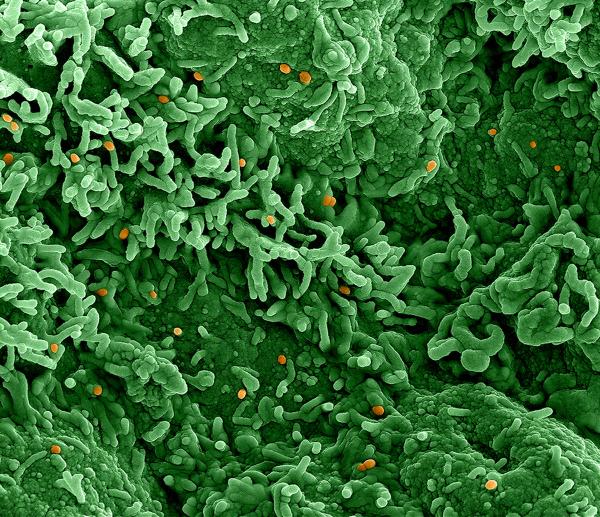
CREDIT: NIAID INTEGRATED RESEARCH FACILITY (FORT DETRICK, MARYLAND)
Colorized scanning electron micrograph of monkeypox virus (orange) on the surface of infected Vero E6 cells (green). The Vero cell line is established from kidney epithelial cells of the African green monkey.
Fast forward to 2022: Since May, the CDC has recorded more than 65,000 cases of monkeypox globally and nearly 24,000 cases in the United States alone. This time, transmission is being driven by human-to-human contact. What makes this outbreak different? The vast majority of cases are affecting interlinked communities of men who have sex with men, although public health officials caution that anyone who has had close contact with an infected person can get the disease.
Vaccines originally developed for smallpox are likely to be effective against monkeypox, too, and scientists are expanding treatment options. But questions remain on precisely how the virus is transmitted, how it might be changing, and what can be done better to combat it. NIH scientists are addressing those gaps in knowledge with new research to improve diagnostics and optimize therapeutics. They are working with colleagues around the globe to understand why this emerging virus is posing new challenges.
The origins of monkeypox outbreaks
In search of monkeypox’s elusive human-animal interface and its natural animal reservoir is Senior Investigator Vincent Munster at the National Institute of Allergy and Infectious Diseases’ (NIAID’s) Rocky Mountain Labs in Hamilton, Montana. His team is studying emerging viruses deep in the rainforest of the Republic of the Congo in Central Africa. Back at the lab, they model how changes in the environment or the virus’s biology itself may affect transmission.

CREDIT: RYAN KISSINGER, NIAID
Vincent Munster’s lab in Rocky Mountain Labs (at NIAID’s Hamilton, Montana, campus) began studying monkeypox in 2017 when an outbreak appeared in a rural logging community in the Republic of the Congo.
“How do you truly get in contact with that particular virus?” said Munster, referring to the conditions needed for a zoonotic virus such as monkeypox to jump the species barrier from animal to human. For example, hunters might be more likely to encounter an infected animal in areas where that animal’s food sources are more abundant. Monkeypox’s natural animal reservoir has yet to be pinpointed, but is widely thought to be rodents, with African rope squirrels, Gambian pouched rats, and African dormice being among the prime suspects.

CREDIT: AUSTIN ATHMAN, NIAID
Postdoctoral visiting fellows in Munster’s lab: Kwe Claude Yinda (left) is doing deep-sequencing analyses on monkeypox samples; Julia Port is involved in experimental animal modeling of monkeypox virus.
The first case of human monkeypox was identified in 1970 in a 9-month-old boy in the Democratic Republic of the Congo (DRC) in Central Africa. Clinically, the disease resembles smallpox, but it’s not as deadly and there are fewer human-to-human transmissions. A virulent strain known as clade one is prevalent in the rainforests of Central Africa, particularly in the DRC where mortality rates frequently top 10%. Fueling the current global outbreak is a subvariant of clade two, which is a milder strain in West Africa with a mortality rate of less than 1%.
Rapidly diagnosing the first cases of an outbreak is a critical step in arresting its advance. In collaboration with African academic and conservation groups, Munster’s Virus Ecology Section (VES) lab has been conducting long-term studies on Ebola and the role of fruit bats, a natural reservoir of the disease. The NIAID team provides training, equipment, and the reagents needed for diagnosis while their partners in Africa provide the laboratory and clinical infrastructure. In 2017, the VES added monkeypox to their list of diagnostic capabilities when an outbreak of that virus appeared in a rural logging community in the Republic of the Congo. “Everywhere you can expect a virus to emerge, we need to ensure that we have appropriate diagnostics,” said Munster, noting that the frequency of small monkeypox outbreaks in Africa had been increasing in recent years.
Improving disease surveillance
Some scientists have suggested that the current monkeypox strain originated from a Nigerian outbreak in 2017 that continued to lurk in the human population before being exported by travelers in recent years. A project led by Ghanaian scientist Irene Owusu Donkor may help shed some light on that theory. Donkor was a postdoctoral fellow in Munster’s lab (2019-2020) and worked on Lassa virus before returning to her native country. She’s now partnering with the VES team on an extensive serosurvey in Ghana to analyze blood samples collected from people across the country before the current monkeypox outbreak began. The presence of poxvirus antibodies in those samples could indicate that there was already widespread circulation and show which parts of the population historically get exposed to monkeypox.

CREDIT: IRENE OWUSU DONKOR
Ghanaian scientist Irene Owusu Donkor (right), a former postdoc in Munster’s lab, and current Munster team member Bob Fischer are performing small-rodent field work in Ghana.
Munster’s lab is also testing the environmental stability of the virus to understand how well it holds up under different conditions. Monkeypox spreads through direct contact with an infected person’s bodily fluids (such as saliva, respiratory droplets, and semen) or via sores or scabs. People are considered infectious from the onset of flu-like symptoms or rash until the scabs have fallen off (typically 2-4 weeks). But scientists don’t yet understand whether it is transmitted by respiratory droplets and how long contaminated surfaces such as bedding remain infectious. Virologists like Munster hope that identifying new routes of transmission will better inform public health messaging.
New diagnostics
More precise diagnostics are needed to enhance epidemiological studies. A team led by Senior Investigator Daniel Douek at NIAID’s Vaccine Research Center (VRC) is developing new monkeypox-specific assays that distinguish between individuals who have been infected with a poxvirus and those who were vaccinated against monkeypox or variola (smallpox). Both viruses are part of the genetically similar Orthopoxvirus genus, as is the less dangerous vaccinia virus, from which today’s poxvirus vaccines are derived.
Orthopoxviruses have a distinct feature: Infection confers cross-immunity to other orthopoxviruses. But for how long, we don’t know. Current blood tests are unable to discriminate between a history of infection and vaccination. A more sensitive assay would help researchers determine to what degree prior vaccination against or infection with any of the orthopoxviruses is protective against monkeypox.
Smallpox preparedness aids monkeypox response
Our arsenal of monkeypox treatments is a result of years of drug development and having stockpiled them for a potential bioterrorist event. Following the 9/11 and anthrax terrorist attacks in 2001, concerns mounted that smallpox could be resurrected and used as a biological weapon. Smallpox was declared eradicated in 1980 following worldwide programs that used a weakened vaccinia vaccine. That vaccine, labeled Dryvax in the United States, was highly effective but could have severe side effects in immunocompromised individuals or those with conditions such as eczema. A safer vaccine that was well tolerated by the entire population was needed.
NIAID subsequently worked with the Biomedical Advanced Research and Development Authority to support the development of a modified vaccinia virus ankara (MVA) vaccine platform known as JYNNEOS. This next-generation vaccine, which is safe for people with weakened immune systems, was approved in 2019 by the United States Food and Drug Administration (FDA) for smallpox and monkeypox. Data from European studies have also shown that it can be used as post-exposure prophylaxis within 4 days and can be considered up to 14 days after exposure due to monkeypox’s long incubation period.
Clinical trials underway
According to Emily Erbelding, director of NIAID’s Division of Microbiology and Infectious Diseases, we still need to learn more about how to improve the tools we already have. JYNNEOS stockpiles are limited, and NIAID-supported trials will determine whether one-fifth the dose administered intradermally (two subcutaneous injections is the standard regimen) results in a safe and effective immune response—a strategy that would stretch the vaccine supply fivefold. That strategy has already been authorized by the FDA for adults and eight trial sites—including one at the NIH Clinical Center—across the country are recruiting volunteers to provide more data on intradermal administration that would support licensure.
The antiviral tecovirimat (Tpoxx), a drug developed with NIAID support, was FDA-approved in 2018 to treat smallpox. Preclinical trials have found the drug has a good safety profile and targets a protein shared by both variola and monkeypox. Recruiting study participants is suddenly easier as a result of the 2022 monkeypox outbreak, and two new NIAID-supported clinical trials in the United States as well as the DRC will test the safety and efficacy of Tpoxx in humans. The trials are expected to provide data on how long people remain infectious whether or not they receive antivirals.
A changing virus
How is monkeypox evolving? NIAID Distinguished Investigator Bernard Moss, chief of the Genetic Engineering Section in the Laboratory of Viral Diseases, is working on figuring that out. Since he came to NIH in 1966, Moss has elucidated every step in the life cycle of pox viruses from cell entry to gene expression to DNA replication and assembly of infectious particles.

CREDIT: CHIA-CHI CHARLIE CHANG, OD
Since coming to NIH in 1966, Bernard Moss has elucidated every step in the life cycle of pox viruses from cell entry to gene expression to DNA replication and assembly of infectious particles. His group is studying the monkeypox virus now.
“Our first interest in monkeypox was to try to understand why the Central African clade was more virulent than the West African clade,” said Moss. His lab uses an animal model known as a castaneous (CAST) mouse, which is highly susceptible to Orthopoxvirus infection, as well as African dormice. In a soon-to-be-released paper, his lab found the Central African clade was significantly more lethal than the West African clade in CAST mice. Studies are now underway to test whether the virulence of the 2022 strains is any different.
Working with monkeypox requires stringent safety precautions. Moss’s Biosafety Level 3 laboratory in Building 33 is specially equipped to conduct research on select agents that have the potential to pose a severe health threat. The facility is monitored by round-the-clock security guards and video surveillance and access requires additional clearances.
Peering into the virus’s genome could alert investigators to the possibility of more dangerous strains. “We would like to learn what genes are important for virulence,” said Moss. So far, his team has discovered many genes from the two clades that are not responsible, and noted that a more complex interaction of multiple genes may be at work.

CREDIT: GEORGE KATSAFANAS, NIAID
Patricia Earl and Jeffrey Americo, members of NIAID’s Genetic Engineering Section, study monkeypox in a Biosafety Level-3 containment laboratory. Photograph taken through a window from outside the lab.
New technologies
Vaccinia was the first virus to be engineered as a recombinant vaccine. Moss, along with his former postdoctoral fellow Enzo Paoletti, were the first to show that a vaccina virus could be modified to protect against other pathogens. Their two papers were published simultaneously (Moss’s paper: Proc Natl Acad Sci USA 79:7415-7419, 1982; Paoletti’s paper: Proc Natl Acad Sci USA 79:4927-4931, 1982). Moss further showed that a recombinant vaccinia virus could protect chimpanzees against hepatitis B (Nature 311:67-69, 1984). Years later, leading up to the development of the JYNNEOS vaccine, Moss and his collaborators published a study demonstrating that the MVA platform could be safely used to protect monkeys from monkeypox (Nature 428:182-185, 2004).
MVA can be difficult to manufacture however, and there are possibilities for new vaccines. Moss is working with Senior Investigator Nancy Sullivan at the VRC in collaboration with the pharmaceutical company Moderna to evaluate the immunogenicity of novel mRNA vaccines for monkeypox.
Current guidance to control the 2022 outbreak has focused on temporarily changing social behaviors while vaccination efforts are ramped up. There are signs that the strategy is working. Case counts have declined since August and behavior surveys show that individuals are taking steps to protect themselves and their partners from monkeypox. But Moss cautions continued vigilance. In 2003 monkeypox did not become widespread. Given the scope of the current outbreak, he warns of the possibility that we might not be so lucky this time. If the virus were to establish itself in the animal population, future outbreaks could become more common.
“One would have to consider that would be a very bad result of this epidemic if monkeypox would resolve in humans but continue as a reservoir in North American animals,” he said.
This page was last updated on Wednesday, November 9, 2022
