Study Identifies How Suppressed HIV Keeps Immune System on Edge
Findings Point to Approaches for Staving Off Health Problems in Infected Individuals
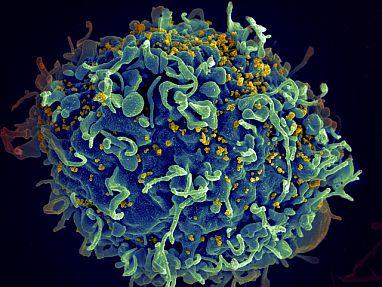
HIV (yellow) needs to activate immune cells like this one to infect them. New IRP research identified how the virus continues to stimulate the immune system even when fully suppressed by antiretroviral therapy.
Over the four decades since it mysteriously began destroying the immune systems of Americans in New York and California, HIV has proven to be a frustratingly wily opponent for scientists. Even today, when treatments can fully suppress the virus in infected individuals, it continues to harm their health. A new IRP study has identified several ways dormant HIV might chronically stimulate the immune system, suggesting potential avenues for preventing the health problems that causes.1
Doctors have been using antiretroviral therapy (ART) to treat HIV for more than 30 years, forcing the virus into an inactive state in the body and turning what was once a death sentence into a manageable, chronic condition. However, over that long time period, they began to notice that people living with successfully suppressed HIV infections were developing age-related ailments like cardiovascular and kidney disease and neurological problems noticeably earlier in life than the general population.
This phenomenon, scientists believe, occurs because HIV infection persistently revs up the immune system, even when ART successfully corrals the virus. Uncontrolled HIV weakens the immune system, leaving patients vulnerable to dangerous, ‘opportunistic’ infections that healthy people easily fend off. However, HIV also stimulates our body’s defenses just like any other virus while it goes about destroying immune cells.

Antiretroviral drugs like these can completely stop HIV from replicating in the human body, but do not stop the dormant virus from activating the immune system.
“HIV can only infect and replicate in activated immune cells, so the more it activates the immune system, the more it creates targets — that’s how it spreads,” says IRP senior investigator Leonid Margolis, Ph.D., the new study’s senior author. "Even when HIV is completely suppressed by antiviral drugs, there is some residual immune activation that remains, and we don’t know what triggers it.”
While there are many theories for why this happens, the lack of good model systems for testing them under controlled laboratory conditions has frustrated efforts to evaluate them. In the new study, Dr. Margolis’ team investigated several of those explanations using an approach they helped develop that allows them to grow tonsil tissue from routine tonsil removal surgeries in their lab.2 Since the tonsils contain large numbers of immune cells, this model provides a good way to study viruses like HIV that infect those cells. Dr. Margolis’ team determined how active the immune cells in the tissue were by measuring their secretion of molecules called cytokines that regulate immune system activity.
More From the IRP
Blog
HIV Research Yields an Unexpected Discovery
Using their model, Dr. Margolis team’ found that ART drugs alone do not rev up immune cells, as some scientists have proposed. Similarly, treating the tissue model with large amounts of cytokines to simulate the extremely amped up immune activation triggered by the early stages of HIV infection — known as a ‘cytokine storm’ — did not cause the tissue to continue over-producing cytokines itself after 12 days had passed. Exposing the tissue to proteins produced by the HIV virus and HIV-infected cells failed to noticeably stimulate the immune cells either.
In contrast, HIV viral particles that the researchers inactivated so they could not infect cells triggered sustained increases in cytokine production in the tissue model. Similarly, the tissue also released more cytokines when it was exposed to tiny capsules called extracellular vesicles released by HIV-infected cells, even when those infected cells had been treated with an antiviral drug.
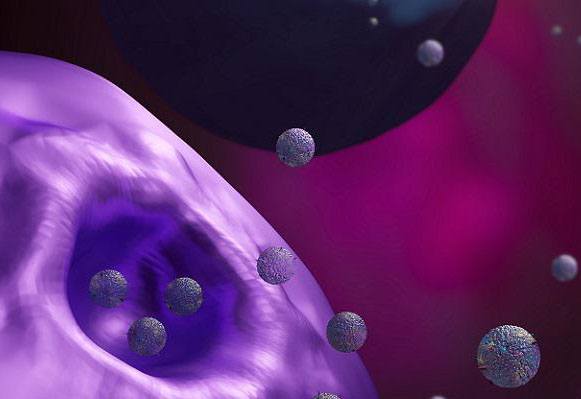
Cells release small sacs of RNA and proteins called extracellular vesicles to communicate with one another.
“These small vesicles mediate cell-cell interactions,” Dr. Margolis explains. “They contain proteins and RNA. They’re the way that cells talk to each other. We don’t know, unfortunately, which infected tissues in the body produce vesicles containing viral proteins and RNA and which don’t. We now know that vesicles from HIV-infected tissue activate the immune system, but we don’t know their source.”
Further experiments revealed that inactivated HIV viruses trip cellular detectors called toll-like receptors (TLRs) present on immune cells, which engage the immune system to respond to invaders. When the IRP researchers treated their tissue model with chemicals that inhibit a particular TLR called TLR8, the inactivated HIV viruses did not spur the tissue to produce extra cytokines.
By identifying the specific mechanisms by which a fully suppressed HIV infection can keep the immune system permanently on edge, Dr. Margolis’ study points towards potential therapeutic approaches for reducing that wayward immune activity. Treatments that accomplish that without broadly weakening the immune system could help stave off the premature appearance of age-related diseases in people living with HIV.
“The general idea of our work is, if you can suppress immune activation, there is a good chance that these diseases would not develop,” Dr. Margolis says. “Of course, it’s very difficult because we need our immune systems to protect us. We need very fine tuning that specifically suppresses immune activation by HIV, but the general immune response should be preserved. For this, we need to know detailed mechanisms.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
References:
[1] Mechanisms of residual immune activation in HIV-1 infected human lymphoid tissue ex vivo. Mercurio V, Fitzgerald W, Vanpouille C, Molodtsov I, Margolis L. AIDS. 2021 Mar 16. doi: 10.1097/QAD.0000000000002881.
[2] Ex Vivo Infection of Human Lymphoid Tissue and Female Genital Mucosa with Human Immunodeficiency Virus 1 and Histoculture. Introini A, Vanpouille C, Fitzgerald W, Broliden K, Margolis L. J Vis Exp. 2018 Oct 12;140:57013. doi: 10.3791/57013.
Related Blog Posts
This page was last updated on Tuesday, May 23, 2023
