An Ebola Therapy Two Decades in the Making
IRP Researcher Nancy Sullivan Led Development of Cutting-Edge Treatment
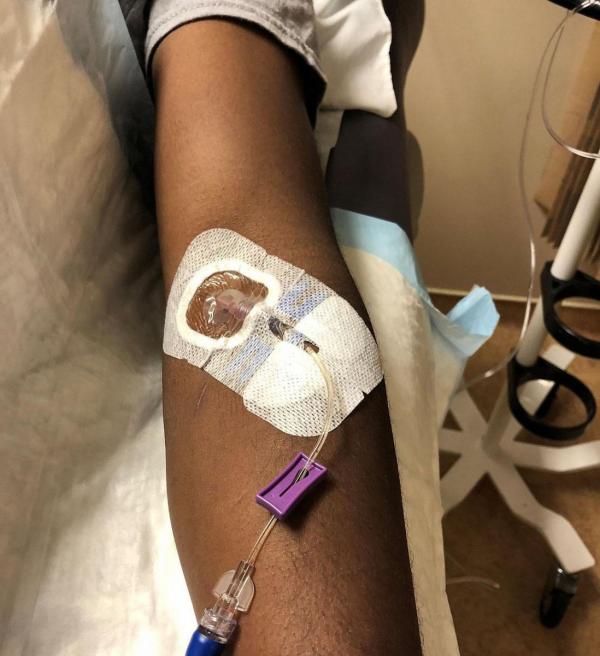
A healthy volunteer receives an IV infusion of an experimental Ebola therapy as part of a phase I clinical trial conducted at the NIH Clinical Center. IRP senior investigator Nancy Sullivan was recently recognized for leading the development of that therapy, one of the first effective treatments for Ebola.
Twenty-four years before the novel coronavirus began spreading in Wuhan, China, an outbreak of another deadly virus burned through the city of Kikwit in what is now the Democratic Republic of Congo. Between January and August of 1995, 316 people are thought to have contracted Ebola, and 252 of them died.1 More than a decade later, a team of NIH infectious disease scientists would track down one of the survivors and use a sample of the individual’s blood to produce one of the first effective treatments for Ebola.
Last spring, IRP senior investigator Nancy J. Sullivan, Ph.D., head of the Biodefense Research Section at the NIH’s Vaccine Research Center (VRC), was named a finalist for the 2020 Samuel J. Heyman Service to America Medals for her role in leading the development of that therapy, which was approved by the FDA in December. Considered the ‘Oscars of government service,’ the awards recognize outstanding achievements by federal employees like Dr. Sullivan.
“I think it’s important that people see that the work being done at the NIH is helpful to public health in the United States and beyond,” says Dr. Sullivan. “Awards like this are really one of the only ways that people see the work that the NIH does.”
At the same time that medical specialists from around the world were working to contain Ebola in Kikwit, Dr. Sullivan was performing her graduate research on HIV. Specifically, she was studying how a protein located on the virus’s surface binds to a ‘receptor’ protein outside the cell in order to infect it, ultimately turning the cell into a factory for producing more of the virus.
“When we think about developing therapies or vaccines for viruses, that binding process is a good one to block,” Dr. Sullivan explains. “It’s the first step for infection, and if you can block that step, you have a pretty good chance of blocking the virus.”
After completing her graduate dissertation, Dr. Sullivan pivoted to studying Ebola because the protein that helps it infect cells looks and behaves similarly to the analogous protein on HIV. As a postdoctoral fellow, she helped develop the first vaccine capable of protecting non-human primates from Ebola. This achievement helped lay the groundwork for other vaccine studies, including the creation of pharmaceutical company Merck’s Ebola vaccine, which became the first of its kind to receive approval from the U.S. Food and Drug Administration (FDA) in December 2019.
The Ebola virus (green) infects a wide variety of cells throughout the body, often leading to lethal consequences.
When she started her own lab at the NIH, Dr. Sullivan began applying her knowledge of vaccine-induced immune responses to the development of therapeutics. Such efforts are critical complements to vaccine research because vaccines do not guarantee immunity and not every at-risk individual will be vaccinated. Dr. Sullivan specifically sought to develop a treatment based on monoclonal antibodies, lab-grown copies of the antibody molecules that the immune system naturally produces to nullify a virus. Her prior research on Ebola vaccines was directly applicable to this endeavor, since vaccines work by stimulating the immune system to produce antibodies. What’s more, that work gave her critical insight into the traits that an antibody would need to neutralize Ebola.
That understanding was all the more important because Ebola is a particularly wily virus. For one thing, the protein that Ebola uses to infect cells has a protective ‘cap’ that helps to shield it from the body’s immune system — and if the immune system can’t detect something, it can’t create antibodies against it. In addition, the cellular receptor the Ebola virus uses to facilitate infection lies deep within the cell, making it a difficult target to block with antibodies that are circulating outside the cell. However, what foils those antibodies fails to stop Ebola. In a clever trick of biology, once inside the cell, the virus uses the cell’s own digestive enzymes to chew off the protective cap, revealing the viral protein that binds to the cell’s receptor and initiating the infection process.
“There will be lots of antibodies that bind to this cap because the cap is on the outside of the virus, but those antibodies are useless once that cap is chewed off,” Dr. Sullivan explains. “So one of our first questions was whether we could even find an antibody that binds to the virus when the cap is on, stays bound as the virus enters the cell, stays bound as the cell’s enzymes chew off the cap, and then blocks receptor binding.”

Image credit: U.S. Centers for Disease Control and Prevention (CDC)
A close-up image of the Ebola virus.
To find such an antibody, Dr. Sullivan’s team examined numerous antibodies isolated from the blood of a patient who survived the 1995 Ebola outbreak in Kikwit. The researchers theorized that a person who beat the virus might have fought it off with the help of an antibody with the features Dr. Sullivan was seeking — an antibody that the survivor’s immune cells might still be able to produce 20 years later. In this endeavor, her experience studying the immune system’s response to Ebola paid dividends once again.
“This is where the basic science was so important,” she says. “It was studying those molecular interactions between the virus and the immune system for many, many years that allowed us to decide which methods to use to identify the antibody we were looking for.”
The specialized techniques that Dr. Sullivan’s team employed identified an antibody, dubbed mAb114, that was highly effective at preventing Ebola from infecting isolated cells in laboratory tests. What’s more, mAb114 protected macaque monkeys from Ebola infection, even when it was administered as late as five days after the animals were exposed to the virus.2

Dr. Sullivan presents her research in a lecture at the NIH.
That work, published in 2016, came at an especially important time for Ebola research. The breakthrough was sandwiched between an Ebola outbreak in West Africa in 2014 and 2015 and another in the Democratic Republic of Congo (DRC) that began in 2018. Consequently, once mAb114 was shown to be effective in non-human primates, the NIH and Dr. Sullivan’s colleagues at the Institut National de la Recherche Biomédicale (INRB), a medical research organization in the DRC, moved quickly to human trials. At the NIH Clinical Center, Martin Gaudinski, M.D, and Julie Ledgerwood, D.O., Chief Medical Officer at the VRC, led a phase I clinical trial that examined the safety of mAb114 in a small group of healthy adults.3 Next, with the therapy proven safe, scientists from the Division of Clinical Research at the NIH’s National Institute of Allergy and Infectious Diseases (NIAID) worked with the World Health Organization and INRB on a clinical trial that compared the effects of mAb114 to three other investigational Ebola therapeutics in patients in the DRC. The trial ultimately found that mAb114 and another monoclonal antibody treatment manufactured by Regeneron Pharmaceuticals dramatically reduced the death rate among patients who received them.4

More From the IRP
Podcast
Dr. Catharine Bosio — The Weird and Deadly Francisella Tularensis Bacterium
While mAb114 was being tested in the DRC, the NIH licensed it to Ridgeback Biotherapeutics, which subsequently applied for FDA approval of the therapy — now under the name Ebanga. Based in part on the results of that clinical trial, the FDA approved both Regeneron’s monoclonal antibody therapy and Ebanga in October and December 2020, respectively, making them the first — and still only — effective antiviral therapies for Ebola.

Dr. Sullivan (far right) gives President Barack Obama a tour of her lab in December 2014. The pair were joined by Dr. Anthony Fauci, Director of the NIH’s National Institute of Allergy and Infectious Diseases (far left), and Department of Health and Human Services Secretary Sylvia Burwell (second from left).
Dr. Sullivan is quick to share the credit for making Ebola treatable for the first time with a long list of IRP colleagues and scientists outside the NIH, including her own trainees; VRC Deputy Director Barney Graham, M.D.; Dr. Ledgerwood’s group in the VRC’s Clinical Trials Program; Dr. Jason Gall’s team at the VRC’s Vaccine Production Program; and the lab of VRC Director John Mascola, M.D. Beyond this extensive teamwork, however, she also recognizes the importance of NIH’s support for the basic research that enabled her to find mAb114 in the first place, as well as the unique resources that the IRP provides to its many scientists.
“Fifteen to twenty years of basic research went into my ability to find the right kind of antibody,” she explains. “That basic research is absolutely critical. If you went out and talked to a non-scientist and told them, ‘NIH is studying the interaction between Ebola GP surface protein and NPC-1 receptor binding,’ most people would yawn and ask why you’re wasting taxpayer money doing that kind of work. We’re lucky that at the NIH we’re able to translate these findings into vaccines and therapeutics.”
That expertise will continue to bear fruit in the future, as IRP scientists continue to pursue research that will be critical to combating a wide array of diseases, including those we have yet to encounter. If nothing else, the saga of mAb114 demonstrates how both the basic and applied research done in the IRP facilitates the rapid development and deployment of approaches to battle deadly illnesses.
“It probably will become even more important now that we’ve had this awakening with COVID-19,” Dr. Sullivan says. “We need to be able to do this and do it quickly; that is, have that research in place already and be able to move something very quickly to testing in humans. That was the original plan when the VRC was established, and I think licensure of mAb114 by the FDA shows that, in fact, the NIH can do that.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
References:
[1] The 1995 Kikwit Ebola outbreak: lessons hospitals and physicians can apply to future viral epidemics. Hall RCW, Hall RCW, Chapman MJ. Gen Hosp Psychiatry. 2008;30(5):446-452. doi: 10.1016/j.genhosppsych.2008.05.003.
[2] Protective monotherapy against lethal Ebola virus infection by a potently neutralizing antibody. Corti D, Misasi J, Mulangu S, Stanley DA, Kanekiyo M, Wollen S, Ploquin A, Doria-Rose NA, Staupe RP, Bailey M, Shi W, Choe M, Marcus H, Thompson EA, Cagigi A, Silacci C, Fernandez-Rodriguez B, Perez L, Sallusto F, Vanzetta F, Agatic G, Cameroni E, Kisalu N, Gordon I, Ledgerwood JE, Mascola JR, Graham BS, Muyembe-Tamfun JJ, Trefry JC, Lanzavecchia A, Sullivan NJ. Science. 2016 Mar 18;351(6279):1339-42. doi: 10.1126/science.aad5224.
[3] Safety, tolerability, pharmacokinetics, and immunogenicity of the therapeutic monoclonal antibody mAb114 targeting Ebola virus glycoprotein (VRC 608): an open-label phase 1 study. Gaudinski MR, Coates EE, Novik L, Widge A, Houser KV, Burch E, Holman LA, Gordon IJ, Chen GL, Carter C, Nason M, Sitar S, Yamshchikov G, Berkowitz N, Andrews C, Vazquez S, Laurencot C, Misasi J, Arnold F, Carlton K, Lawlor H, Gall J, Bailer RT, McDermott A, Capparelli E, Koup RA, Mascola JR, Graham BS, Sullivan NJ, Ledgerwood JE; VRC 608 Study team. Lancet. 2019 Mar 2;393(10174):889-898. doi: 10.1016/S0140-6736(19)30036-4.
[4] A Randomized, Controlled Trial of Ebola Virus Disease Therapeutics. Mulangu S, Dodd LE, Davey RT Jr, Tshiani Mbaya O, Proschan M, Mukadi D, Lusakibanza Manzo M, Nzolo D, Tshomba Oloma A, Ibanda A, Ali R, Coulibaly S, Levine AC, Grais R, Diaz J, Lane HC, Muyembe-Tamfum JJ; PALM Writing Group, Sivahera B, Camara M, Kojan R, Walker R, Dighero-Kemp B, Cao H, Mukumbayi P, Mbala-Kingebeni P, Ahuka S, Albert S, Bonnett T, Crozier I, Duvenhage M, Proffitt C, Teitelbaum M, Moench T, Aboulhab J, Barrett K, Cahill K, Cone K, Eckes R, Hensley L, Herpin B, Higgs E, Ledgerwood J, Pierson J, Smolskis M, Sow Y, Tierney J, Sivapalasingam S, Holman W, Gettinger N, Vallée D, Nordwall J; PALM Consortium Study Team. N Engl J Med. 2019 Dec 12;381(24):2293-2303. doi: 10.1056/NEJMoa1910993.
Related Blog Posts
This page was last updated on Tuesday, January 30, 2024
