Antibody Assault Shows Promise Against Dormant HIV
Treatment Strategy Could Mop Up Virus Hiding in Patients’ Bodies
IRP researchers have shown that a specially-designed antibody can unmask cells harboring inactive HIV and lead to their destruction.
As temperatures drop in the fall, a variety of species from groundhogs to bears prepare to wait out the winter in their dens. Much like these animals, HIV also goes into hibernation when conditions are tough, a trait that has long stymied efforts to develop a cure. However, IRP researchers recently tested a promising strategy that might one day be used to flush out and kill dormant remnants of HIV.1
Modern antiretroviral therapy thoroughly wipes out actively replicating HIV in patients’ blood, but unfortunately, the wily virus can protect itself by holing up inside CD4+ T cells, the variety of immune cell it infects. This ‘latent’ HIV can remain dormant indefinitely, waiting to spring back into action if its host ever stops taking antiretroviral medications.
“You don’t actually cure anybody with antiretroviral therapy,” explains IRP senior investigator Richard Koup, M.D., the new study’s senior author. “Obviously, if someone tolerates the medications very well and doesn’t have side effects, it’s a very reasonable therapy to maintain suppression. Having said that, there are a number of individuals who have severe side effects or because of their lifestyles aren’t able to take a pill a day or see a doctor regularly, in which case the HIV may not be controlled.”
The key to eliminating latent HIV is to trick it into revealing itself, kind of like kicking a hornet’s nest. In fact, this strategy is sometimes called ‘kick and kill.’ Once latent HIV starts replicating inside a CD4+ T cell, the cell begins producing markers that flag it as infected. At that point, clinicians must stimulate the immune system so that other immune cells, called CD8+ T cells, can kill those cells. Meanwhile, antiretroviral therapy must be maintained to kill newly generated HIV that escapes into the blood and prevent it from infecting new cells.
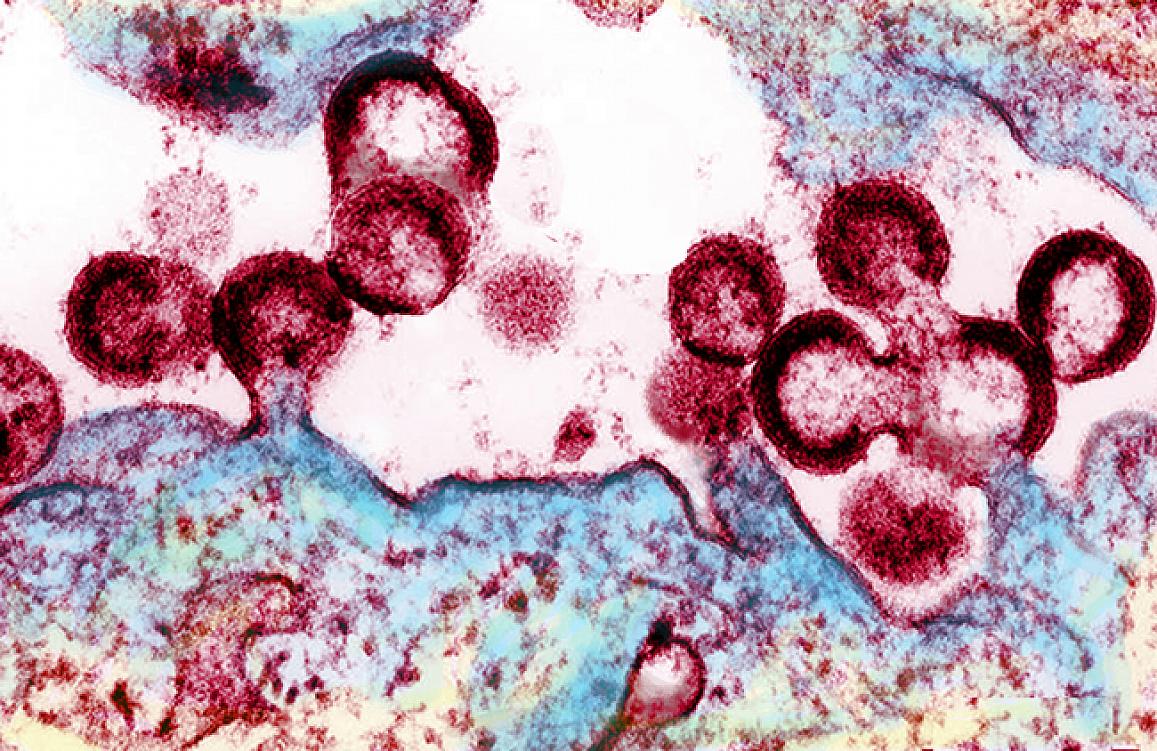
HIV viruses (red) emerging from an infected cell. Counterintuitively, any strategy that seeks to eliminate latent HIV must first reactivate it, causing it to replicate inside infected cells and release into the blood, where the virus will be destroyed by antiretroviral medications.
Current ‘kick-and-kill’ strategies can effectively wake up dormant HIV, but they need something added to them to activate CD8+ T cells. Consequently, Dr. Koup’s lab has been looking for an ‘antibody’ molecule that can both ‘kick’ latent HIV into gear and stimulate CD8+ T cells to ‘kill’ infected cells. Based on the new study’s results, they may have found one in a three-armed ‘trispecific’ antibody developed by ModeX Therapeutics, which leveraged the IRP’s research facilities and the expertise of Dr. Koup’s team to examine the antibody’s effects on isolated cells with latent HIV.
Infected cells containing actively replicating HIV produce molecular flags that antibody molecules can bind to. This allows the antibody tested in the new IRP study to guide CD8+ T cells to the right targets.
The ModeX antibody is an updated version of a two-armed antibody Dr. Koup’s team had previously tested.2 One site on that antibody triggers a cellular switch called CD3 that activates both CD4+ T cells and CD8+ T cells. Any latent HIV inside the activated CD4+ T cells will then start replicating, producing molecular flags that mark them as a target for the newly activated CD8+ T cells. The antibody also binds to those same molecular flags, allowing it to bring CD8+ T cells together with the infected CD4+ T cells that they need to destroy. The newer version of the antibody that Dr. Koup’s team tested in the more recent study also has a third site that activates both types of immune cells in another way, through a receptor called CD28, thereby boosting its potency. What’s more, this antibody strategy can recruit CD8+ T cells in the body’s war on HIV that would normally remain on the sidelines.
“Usually, CD8+ T cells only kill cells they have been pre-programmed to kill,” Dr. Koup explains. “Normally, only an HIV-specific CD8+ cell can kill a CD4+ cell infected with HIV. With this strategy, any CD8+ cell that gets activated will now be able to kill CD4+ cells that are producing HIV. You’re non-specifically activating the CD8+ cell and you’re putting it right next to a CD4+ cell that’s producing HIV, and the CD8+ cell kills it.”
Indeed, when CD8+ T cells from healthy people were put in petri dishes with cells containing either active HIV or dormant HIV, the trispecific antibody caused the CD8+ T cells to kill the infected cells. These experiments also confirmed that the antibody causes as much as 22 times more destruction of infected cells when it has the CD28-targeting site in addition to the other two sites, compared to when it does not trigger CD28. In addition, although the antibody effectively activated both CD4+ and CD8+ T cells even when it could not bind to the molecular flags on CD4+ T cells containing active HIV, that version of the antibody did not lead to much destruction of infected cells.
Promisingly, giving the antibody to healthy primates did not cause any problematic side effects. Those studies also suggested that administering the antibody via a ‘subcutaneous’ injection under the skin is preferable to delivering it ‘intravenously’ directly into the bloodstream.

Dr. Richard Koup
“The intravenous route worked very quickly and gave the highest level, initially, of immune activation, but if you want a prolonged activation, subcutaneous administration was the best,” Dr. Koup says. “What we’re worried about in any of these therapies where you’re giving an immune system activator is that activating a lot CD4+ and CD8+ T cells all at once can be extremely toxic. You want to stimulate a tolerable level of immune activation over a longer period of time, and the subcutaneous route actually works the best for doing that.”
Of course, much more research is needed before the antibody used in the study, or a similar one, can be used to rid people of dormant HIV infections. Dr. Koup’s lab, for instance, plans to examine the longer-term effects of the antibody in monkeys with a primate version of HIV. Although he admits that he is unsure whether ModeX’s antibody will ultimately lead to a cure for HIV, he emphasizes that if a cure is possible, the antibody is, at the very least, an important stepping stone to reaching it.
“You’ve got to crawl before you can walk and walk before you can run,” he says. “The more we learn about latent HIV through these types of studies, the closer we’re going to get to understanding what we’re ultimately going to need.”
“Cure is the ultimate goal that we’re looking for,” he adds. “We’ve achieved it with other viral infections, and I think it makes sense to push forward with that for HIV.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
References:
[1] Promsote W, Xu L, Hataye J, Fabozzi G, March K, Almasri CG, DeMouth ME, Lovelace SE, Talana CA, Doria-Rose NA, McKee K, Hait SH, Casazza JP, Ambrozak D, Beninga J, Rao E, Furtmann N, Birkenfeld J, McCarthy E, Todd JP, Petrovas C, Connors M, Hebert AT, Beck J, Shen J, Zhang B, Levit M, Wei RR, Yang ZY, Pegu A, Mascola JR, Nabel GJ, Koup RA. Trispecific antibody targeting HIV-1 and T cells activates and eliminates latently-infected cells in HIV/SHIV infections. Nat Commun. 2023 Jun 22;14(1):3719. doi: 10.1038/s41467-023-39265-z.
[2] Pegu A, Asokan M, Wu L, Wang K, Hataye J, Casazza JP, Guo X, Shi W, Georgiev I, Zhou T, Chen X, O'Dell S, Todd JP, Kwong PD, Rao SS, Yang ZY, Koup RA, Mascola JR, Nabel GJ. Activation and lysis of human CD4 cells latently infected with HIV-1. Nat Commun. 2015 Oct 20;6:8447. doi: 10.1038/ncomms9447.
Related Blog Posts
This page was last updated on Tuesday, November 14, 2023
