HIV Research Yields an Unexpected Discovery
A Conversation with Dr. Paolo Lusso

IRP senior investigator Dr. Paolo Lusso
First discovered in 1981, human immunodeficiency virus, or HIV, caused one of the most deadly and persistent epidemics in history. HIV destroys CD4+ T cells, a type of white blood cell essential for fighting infection. In doing so, HIV destroys the body’s ability to fight off disease, which often leads to life-threatening consequences.
Today, medications have allowed people living with HIV to lead healthier lives. However, HIV still remains a major public health concern and continues to be studied by researchers within the IRP and beyond.
IRP research has produced findings essential to the development of current HIV treatments and tools for diagnosis. However, there is still a lot left to learn. One recent IRP contribution to HIV research was a 2017 study led by IRP senior investigator Paolo Lusso, M.D., Ph.D., which suggests that treatments targeting a protein called integrin α4β7 could potentially become an addition to current treatment options for those with HIV, or provide new measures to prevent infection.
In honor of National HIV Testing Day today, we spoke with Dr. Lusso to discuss this HIV study, what it could mean for future treatments, and his continued efforts in the fight against the illness.
What prompted you to conduct this research?
“First, about 20 years ago the field of HIV study began to recognize the importance of the intestinal compartment. Although the intestines are not typically associated with the immune system, research found that it is actually at the center stage of HIV infection and disease. Where historically there was low emphasis on the intestines, it started to become understood that they are one of the most important immune system organs due to the high number of lymphocytes [disease-fighting white blood cells] that reside in the gut-associated lymphoid tissue (GALT) to protect the body and gut. Looking back, this makes perfect sense because the gut is a very active border between what is inside and outside the body.
“The gut is an area which understandably has many ‘soldiers’ of the immune system that are continuously circulating in and out. Because the main cells targeted by HIV, the CD4+ T cells, live in the gut in great numbers, the gut is one of the major sites where HIV infection replicates and destroys healthy CD4+ T cells. Therefore, we believe the gut is one of the first sites where HIV begins damaging the immune system, and this is one of the reasons I was attracted to study in-depth the connection between HIV and the gut.
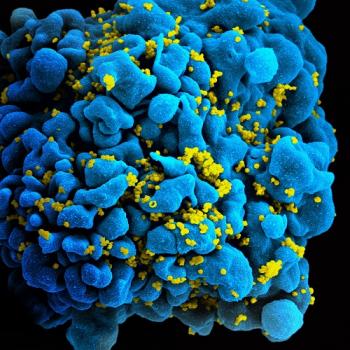
The HIV virus (yellow) weakens the immune system by infecting and killing T cells like this one (blue).
“The second reason was related to the main ‘actor’ in our [2017] paper, α4β7, which is one of the major systems that attracts cells to the gut. Some cells have an ‘address’ stamped on their surface that regulates where they are sent to, and α4β7 is the address that sends cells to the gut. In fact, a large number of CD4+ T cells spend the majority of their time in the gut and have the α4β7 address stamp, called a ‘gut-homing receptor.’
“At the time, it was known that the CD4+ T cells were the main target cells for the HIV virus, which uses the protein they’re named for, CD4, as a port-of-entry. However, a few years ago, other scientists in my lab at the NIH discovered that α4β7 can stick to the surface of HIV, allowing HIV to use the α4β7 molecule as an accessory receptor. As a result, there was a lot of interest in α4β7 in the gut as a major site for HIV infection, replication, and cell destruction within our lab.
“We actually jumped into the study out of a little serendipity because we were initially asking a different question. At first, we wanted to see if HIV infections were actually changing the expression of α4β7 over time. When we started the study, we exposed cells to HIV and studied the expression of α4β7 on the surface of the infected cells. To our surprise we saw a significant increase of α4β7 expression in these studies. From there we started investigating if this expression was happening over a period of time or right away. We then found that this change was happening in a matter of minutes and thus was not something that was generated inside the cell; it was clearly something brought upon by the virus itself.
“Following the studies, we started to devise new tests and strategies that definitively proved that the HIV virus does contain α4β7, which is a cellular protein and not a viral protein, and that’s how the whole thing started.”
What impact has this research had to-date?
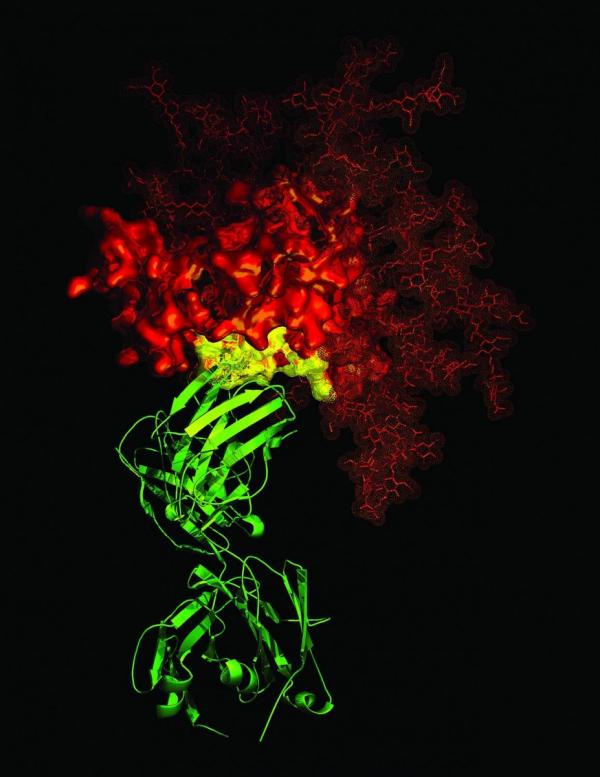
An antibody (green) attached to a protein on the surface of the HIV virus (red)
“After we discovered that the HIV virus carries α4β7, we proposed that if α4β7 is present on the virus, one could introduce an antibody that attaches to the molecule and the effect would be an immune complex created by the combination of the virus and antibody.
“Immune complexes are known to be a very good method of stimulating the immune system and some people have even started to use immune complexes for vaccination. Recently, in collaboration with [IRP senior investigator and NIAID Director] Dr. Anthony Fauci, we actually began an HIV vaccine study using immune complexes to determine if we can induce better, more effective immune responses in animal models and potentially protect the animals from the infection or from developing the disease.
“We also believe that α4β7 is important in sexual transmission of HIV and is probably a factor in facilitating the infection. Because the virus is bringing α4β7 along on its surface, we believe this could help the virus gain access into the body.
“To prove this, we recently started a study where we have produced viruses that contain or do not contain α4β7. From there we’re planning to test to see if the virus with α4β7 is more readily transmissible.
“As we continue the study we’re hoping to receive more information on possible new ways to vaccinate against HIV. If the data shows that α4β7 is important for HIV transmission, we can then use drugs or antibodies to protect people before they even get the initial infection.”
What was the most challenging aspect of this research?
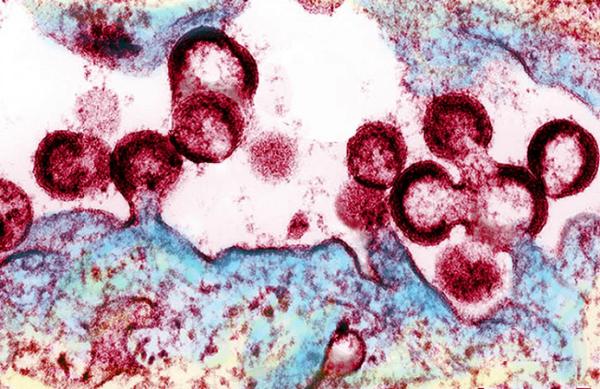
HIV viruses releasing from an infected cell
“One of the most important mental biases that a scientist always has is, ‘How true will what we’re observing be in real life?’ There was no question that we saw α4β7 on the virus. We tested many different strains which all incorporated α4β7. We also tested to see if it was functional, so we had a lot of really nice and convincing data. However, this was all in a test tube and you start to wonder how important this phenomenon you see in the lab is in real life because this is where a lot of research fails. As scientists, at some point we wonder how these test-tube and animal studies really translate into humans.
“In our research, we then started to study human samples, which was a little more challenging because we needed to obtain suitable samples. Because α4β7 incorporation is more efficient in the early phase of infection, it was difficult to obtain samples as many people don’t even realize they have HIV at this phase. However, we did obtain them, and when we tested them we were very excited because the testing was completely in agreement with our test-tube results: even real-life virus directly taken from the blood of patients contained α4β7! More recently, we received semen samples from individuals recently diagnosed with HIV, which we’re now ready to study to see if we can show that the virus in the samples has α4β7. If so, it might be a very important validation of our model showing that α4β7 can help the virus get into a person's body.”
How are you planning to expand on this research in the future?
“On top of the ongoing studies, we would really like to move past the experimental models into the human system and, at some point, perform clinical trials. We’re ready to collect data from the animal studies and will hopefully get some answers. If the results are positive, we’ll move as quickly as possible to begin in the human system. At that point, we will potentially be able to start a clinical study that directly addresses the question of transmission or the possibility of vaccinating people with an immune complex as a way to create a stronger immune response to protect individuals from HIV, or at least better control the virus if they’re already infected. That is the ultimate step.”
Head over to our Accomplishments page for more information on Dr. Lusso's research, and subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
Related Blog Posts
This page was last updated on Tuesday, May 23, 2023
