Simplifying HIV Treatment: A Surprising New Lead
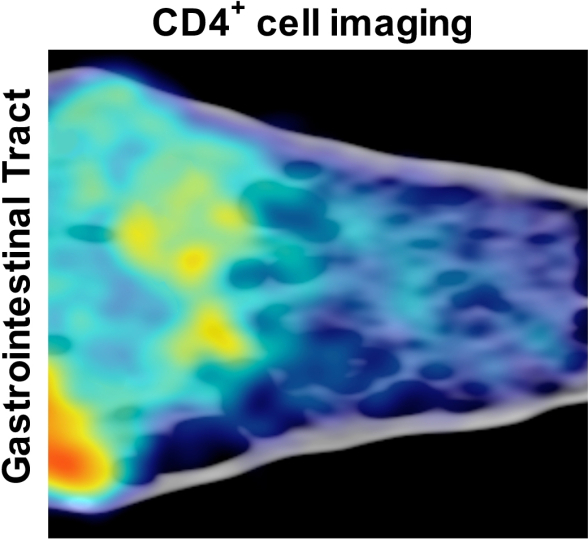
Credit: Byrareddy et al., Science (2016).
PET/CT imaging reveals a surprisingly high concentration (yellow, light green) of key immune cells called CD4 T cells in the colon (left) of an SIV-infected animal that received antibody infusions along with antiviral treatment. Fewer immune cells were found in the small intestine (right), while the liver (lower left) shows a high level of non-specific signal (orange).
The surprising results of an animal study are raising hopes for a far simpler treatment regimen for people infected with the AIDS-causing human immunodeficiency virus (HIV). Currently, HIV-infected individuals can live a near normal life span if, every day, they take a complex combination of drugs called antiretroviral therapy (ART). The bad news is if they stop ART, the small amounts of HIV that still lurk in their bodies can bounce back and infect key immune cells, called CD4 T cells, resulting in life-threatening suppression of their immune systems.
Now, a study of rhesus macaques infected with a close relative of HIV, the simian immunodeficiency virus (SIV), suggests there might be a new therapeutic option that works by a mechanism that has researchers both excited and baffled [1]. By teaming ART with a designer antibody used to treat people with severe bowel disease, NIH-funded researchers report that they have been able to keep SIV in check in macaques for at least two years after ART is stopped. More research is needed to figure out exactly how the new strategy works, and whether it would also work for humans infected with HIV. However, the findings suggest there may be a way to achieve lasting remission from HIV without the risks, costs, and inconvenience associated with a daily regimen of drugs.
Left untreated, both SIV and HIV attack and destroy CD4 T cells. Previous studies have shown that these viruses preferentially target CD4 T cells expressing high levels of a particular integrin receptor, called α4β7, on their surfaces. The receptor has also been thought to act as a kind of “zipcode” that routes CD4 T cells to the gastrointestinal tract, where they serve as a reservoir for HIV replication.
In a study published in the journal Science, researchers at Emory University School of Medicine, Atlanta, and NIH’s National Institute for Allergy and Infectious Diseases set out to explore whether response to ART might be improved by interfering with CD4 T cells that express the key integrin receptor. The agent they chose to run such interference was an antibody similar to vedolizumab, an integrin receptor-blocking antibody drug recently approved by the Food and Drug Administration for treatment of two severe intestinal diseases: ulcerative colitis and Crohn’s disease. Researchers hypothesized that this antibody might be able to prevent CD4 T cells from homing in on the gastrointestinal tract, thereby helping to control the virus better.
To test this theory, the researchers turned to the SIV-infected rhesus macaques. Eighteen macaques received daily ART treatment for about 3 months. A month into the ART regimen, 11 of the animals also began receiving infusions of an antibody similar to vedolizumab every 3 weeks and those treatments lasted about 6 months. The remaining seven animals got infusions of a non-specific antibody as a control.
As soon as the ART was stopped, SIV levels rose in the controls. In contrast, eight of the animals that received the vedolizumab-like antibody demonstrated a remarkable and lasting ability to control SIV. In fact, researchers report that levels of virus in their bloodstream have remained at low or undetectable levels for two years and counting. (An important safety note: three of the animals receiving the antibody developed immune reactions to the antibody drug, forcing their withdrawal from the study).
As you can see in the image above, the researchers used a novel PET/CT imaging technique to track CD4 T cells within the macaques. That imaging showed that the animals’ immune systems improved after the ART-antibody combo treatment. And it also revealed a big surprise: animals treated with vedolizumab-like antibody had almost normal CD4 T cell levels in their intestines. In other words, the new treatment strategy had worked—but probably not in the way the researchers had anticipated!
What’s more, SIV control in the macaques wasn’t explained by the production of broadly neutralizing antibodies (bnAbs). That is noteworthy because bnAbs are known to protect a small fraction of HIV-infected people after their immune systems have battled the virus for many years. Researchers working to develop an effective HIV vaccine have struggled for years to find ways to coax the immune system to produce those bnAbs more quickly. The new findings therefore suggest there may be another, as yet unidentified, target for HIV vaccines, as well as treatments.
It must be noted that the researchers initiated ART in the SIV-infected animals within weeks of infection, which may be difficult to achieve in HIV-infected people. If the virus was provided with additional time to take up residence in CD4 cells in the intestinal tract, the vedolizumab-like antibody might be less likely to work. Clearly, more research is needed to solve the mystery of how ART-antibody treatment acts to keep the lid on SIV over the long term, and whether it could work similarly against HIV.
We likely won’t have to wait long for more insights, however. A small, early phase human clinical trial is already underway at the NIH Clinical Center, Bethesda, MD to test whether a 30-week course of vedolizumab is safe and effective in HIV-infected volunteers. Preliminary results are expected by the end of 2017.
Reference:
[1] Sustained virologic control in SIV+ macaques after antiretroviral and α4β7 antibody therapy. Byrareddy SN, Arthos J, Cicala C, Villinger F, Ortiz KT, Little D, Sidell N, Kane MA, Yu J, Jones JW, Santangelo PJ, Zurla C, McKinnon LR, Arnold KB, Woody CE, Walter L, Roos C, Noll A, Van Ryk D, Jelicic K, Cimbro R, Gumber S, Reid MD, Adsay V, Amancha PK, Mayne AE, Parslow TG, Fauci AS, Ansari AA. Science. 2016 Oct 14;354(6309):197-202.
Links:
- HIV/AIDS Basics (AIDS.gov)
- HIV/AIDS (National Institute of Allergy and Infectious Diseases/NIH)
NIH Support: National Institute of Allergy and Infectious Diseases; Eunice Kennedy Shriver National Institute of Child Health and Human Development; Office of the Director
Related Blog Posts
This page was last updated on Tuesday, May 23, 2023
