Toward an AIDS-Free Generation: Can Antibodies Help?
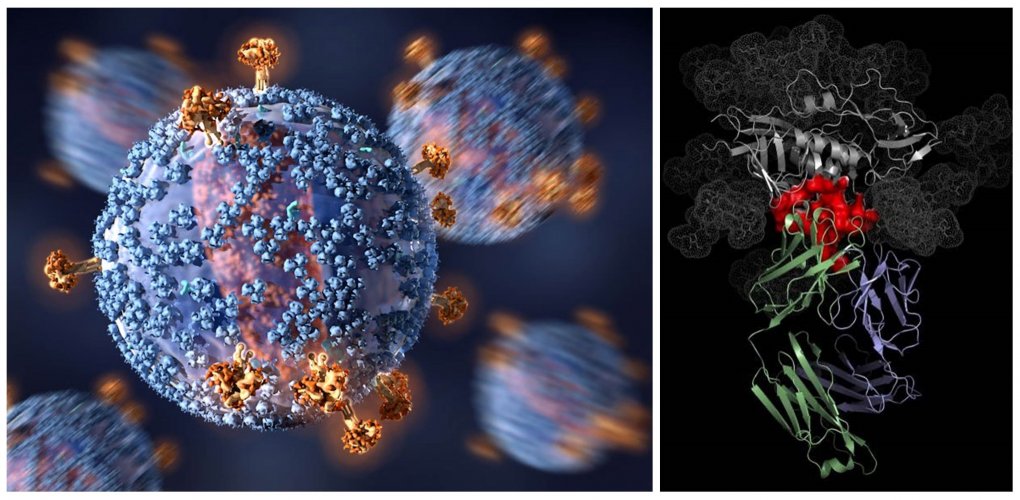
Credits: C. Bickel, Science Translational Medicine; National Institute of Allergy and Infectious Diseases
Left: Human Immunodeficiency Virus (HIV); Right: VRC01 antibody (blue and green) binding to HIV (grey and red). The VRC01-HIV binding (red) takes place where the virus attaches to primary immune cells.
This year, an estimated 50,000 Americans will learn they have been newly infected with the human immunodeficiency virus (HIV), which causes AIDS [1]. The good news is that if these people are diagnosed and receive antiretroviral therapy (ART) promptly, most will enjoy a near-normal lifespan. The bad news is that, barring any further research advances, they will have to take ART every day for the rest of their lives, a regimen that’s inconvenient and may cause unpleasant side effects. Clearly, a new generation of safe, effective, and longer-lasting treatments to keep HIV in check is very much needed.
That’s why I’m encouraged to see some early signs of progress emerging from a small, NIH-supported clinical trial of an HIV-neutralizing antibody. While the results need to be replicated in much larger studies, researchers discovered that a single infusion of the antibody reduced levels of HIV in the bloodstreams of several HIV-infected individuals by more than 10-fold [2]. Furthermore, the study found that this antibody—known as a broadly neutralizing antibody (bNAb) for its ability to defend against a wide range of HIV strains—is well tolerated and remained in the participants’ bloodstreams for weeks.
While the human immune system is generally unable to fend off HIV—in part because the virus tends to mutate as it multiplies—a minority of infected individuals eventually do produce bNAbs with the ability to target the virus in its many forms. The antibody featured in the new study, called VRC01, is a manufactured version of a protein identical to one that researchers from NIH’s National Institute of Allergy and Infectious Diseases (NIAID) isolated from the blood of an infected patient back in 2009 [3]. The NIAID team went on to show that VRC01 and a related antibody could stop more than 90 percent of known HIV strains from infecting human cells in the laboratory.
The secret to VRC01’s power to fight off many HIV strains is its ability to target one of a few conserved areas on the viral surface [4]. This discovery has inspired efforts to devise a vaccine that could train the immune system to make antibodies with a similarly broad neutralizing ability. It also raised the possibility that direct infusions of bNAbs might benefit HIV-infected people unable to produce these antibodies themselves.
To test this idea, researchers at the NIAID’s Vaccine Research Center led by Julie Ledgerwood and John Mascola evaluated VRC01 in 23 HIV-infected people, including 15 who were taking ART and eight who were not. Those treated with ART received two VRC01 infusions 28 days apart. Those with untreated HIV received a single infusion. The average participant was 35 years old, most were college educated, and about 80 percent were male.
In a study published in the journal Science Translational Medicine, researchers reported that the antibody treatment proved safe in all participants. While the antibody didn’t appear to lower levels of HIV inside of blood cells, it did significantly reduce levels of free-floating virus in the bloodstreams of six of the eight ART-untreated individuals. The reductions, which ranged from 12-to-59 fold, persisted for an extended period of time. What’s more, in two of these six, the antibody reduced free-floating HIV to undetectable levels for about three weeks—or as long as VRC01 remained at therapeutic concentrations.
As for the two ART-untreated individuals who didn’t respond to the antibody, they were found to carry viral strains that were more resistant or less sensitive to VRC01. Also, the antibody didn’t have any apparent added benefit for 15 participants who were already taking effective ART.
While promising, the findings suggest it will take more than VRC01 alone to treat chronic HIV infection. It’s possible the antibody could have a more potent effect in people who are newly infected with the virus, and another early phase trial to test this notion is set to begin soon in sub-Saharan Africa [5]. There’s also evidence that VRC01 infusions delivered prior to HIV exposure might prevent infection. Clinical studies of VRCO1 infusion in healthy adults at high risk of infection are expected to begin this year in the U.S. and sub-Saharan Africa [6], and an early phase safety study in exposed infants is currently recruiting patients in locations around the world [7].
There’s a long road still ahead in the quest to develop more effective means to treat and prevent HIV. But, with continued advances such as these, the path toward our ultimate goal is finally coming into view: a world in which new HIV infections are rare and deaths due to AIDS are even rarer.
References:
[1] HIV/AIDS: Basic Statistics. Centers for Disease Control and Prevention. 2015.
[2] Virologic effects of broadly neutralizing antibody VRC01 administration during chronic HIV-1 infection. Lynch RM, Boritz E, Coates EE, DeZure A, Madden P, Costner P, Enama ME, Plummer S, Holman L, Hendel CS, Gordon I, Casazza J, Conan-Cibotti M, Migueles SA, Tressler R, Bailer RT, McDermott A, Narpala S, O’Dell S, Wolf G, Lifson JD, Freemire BA, Gorelick RJ, Pandey JP, Mohan S, Chomont N, Fromentin R, Chun TW, Fauci AS, Schwartz RM, Koup RA, Douek DC, Hu Z, Capparelli E, Graham BS, Mascola JR, Ledgerwood JE; VRC 601 Study Team. Sci Transl Med. 2015 Dec 23;7(319):319ra206.
[3] Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Wu X, Yang ZY, Li Y, Hogerkorp CM, Schief WR, Seaman MS, Zhou T, Schmidt SD, Wu L, Xu L, Longo NS, McKee K, O’Dell S, Louder MK, Wycuff DL, Feng Y, Nason M, Doria-Rose N, Connors M, Kwong PD, Roederer M, Wyatt RT, Nabel GJ, Mascola JR. Science. 2010 Aug 13;329(5993):856-861.
[4] Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Zhou T1, Georgiev I, Wu X, Yang ZY, Dai K, Finzi A, Kwon YD, Scheid JF, Shi W, Xu L, Yang Y, Zhu J, Nussenzweig MC, Sodroski J, Shapiro L, Nabel GJ, Mascola JR, Kwong PD. Science. 2010 Aug 13;329(5993):811-817.
[5] Safety and Virologic Effect of a Human Monoclonal Antibody (VRC01) Administered Intravenously to Adults During Early Acute HIV Infection. Clinicaltrials.gov.
[6] Evaluating the Safety and Efficacy of the VRC01 Antibody in Reducing Acquisition of HIV-1 Infection. Clinicaltrials.gov.
[7] Evaluating the Safety and Pharmacokinetics of VRC01, a Potent Anti-HIV Neutralizing Monoclonal Antibody, in HIV-1-Exposed Infants. Clinicaltrials.gov.
Links:
- HIV/AIDS Basics (AIDS.gov)
- Vaccine Research Center (National Institute of Allergy and Infectious Diseases/NIH)
NIH Support: National Institute of Allergy and Infectious Diseases; National Cancer Institute
Related Blog Posts
This page was last updated on Tuesday, May 23, 2023
