IRP’s Robert Tycko Elected to National Academy of Sciences
NIH Researcher Recognized for Contributions to Structural Biology Techniques

IRP researcher Robert Tycko was elected to the National Academy of Sciences in 2020 for his important contributions to the field of structural biology.
The National Academy of Sciences (NAS), established in 1863, is comprised of the United States’ most distinguished scientific scholars, including nearly 500 Nobel Prize winners. Members of the NAS are elected by their peers and entrusted with the responsibility of providing independent, objective advice on national matters related to science and technology in an effort to advance innovations in the United States.
IRP senior investigator Robert Tycko, Ph.D., was one of two NIH researchers elected to the NAS in 2020, an honor he hopes will give him the opportunity to help other scientists and improve the way science is done.
Dr. Tycko serves as deputy chief of the Laboratory of Chemical Physics in the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), where his work focuses primarily on the development of new methods and applications for solid-state nuclear magnetic resonance (NMR) spectroscopy, a technique that can be used to determine the chemical composition and molecular structure of many different types of materials, as well as how their molecules move around. By measuring the distances between atoms in a structure and feeding those measurements into a computer program, solid-state NMR enables scientists to create a 3-D representation of the protein and infer important information about its shape and structure. That data can, in turn, be used to better understand how that protein may behave, its role and impact in biological processes and diseases, and possibly how it can be targeted with therapeutics.
Dr. Tycko’s research group applies solid-state NMR to the study of proteins and protein assemblies involved in diseases and other biological processes. In particular, his lab uses solid-state NMR and other technologies, such as electron microscopy, to learn more about amyloid-beta fibrils, strings of aggregated proteins that comprise the plaques found in the brain in Alzheimer’s disease and Parkinson’s disease. Amyloid fibrils in the pancreas are also thought to play a role in type 2 diabetes.

Scientists use NMR magnets like this one to learn about the structure of many kinds of molecules.
When Dr. Tycko began this work in 1998, there was “very little known about the molecular structures of amyloids,” Dr. Tycko says. One reason for this was the dearth of biologists using solid-state NMR at the time. Due to the solid structure of amyloid protein assemblies, common analytical technologies like x-ray crystallography and liquid-state NMR weren’t useful — amyloid aggregates do not have a crystalline structure and cannot be dissolved in a liquid.
“But it turns out that we can study amyloid fibrils using solid-state NMR,” Dr. Tycko explains. “My lab did some of the early solid-state NMR studies of amyloid fibrils and we were the first to demonstrate that you could actually determine complete molecular structures of amyloid fibrils from experimental data.”
Dr. Tycko’s experience with solid-state NMR came from more technological and industrial applications while in graduate school at the University of California, Berkeley, during a post-doctoral fellowship at the University of Pennsylvania, and while working in physics and materials science at AT&T’s Bell Laboratories.
“While I was at Bell Laboratories, I started to realize that the techniques I was working on could potentially have useful applications in biological systems,” Dr. Tycko says. “I had in mind that eventually I would start working on biological problems.”
The opportunity to do just that came in 1994. Having recently married and become a father, Dr. Tycko and his family moved to Maryland to start a new life together. He was particularly drawn to the freedom the NIH Intramural Research Program offers to young researchers to explore their fields. Confident in the flexibility allowed by his new employer and the potential of solid-state NMR to solve pressing problems in biology, Dr. Tycko arrived at the NIH with a vast array of possible projects to pursue.
“I knew I wanted to work on some biological problems, but I didn't know which ones,” Dr. Tycko explains. “I think that's one of the great things about the Intramural Research Program: you have a lot of freedom to explore new research directions.”
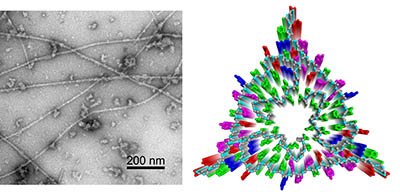
Transmission electron microscope image of amyloid-beta fibrils derived from human Alzheimer's disease brain tissue (left) and cross-sectional view of a molecular structural model for these fibrils based on solid-state NMR data (right).
Applying solid-state NMR to the study of biological molecules was a fairly new endeavor at the time, although it has become much more common in the years since. In the 1990s, only a handful of labs were using the technology for that purpose worldwide, and there weren’t yet many instances in which the measurements had proved important. Dr. Tycko’s lab bucked that trend; by inventing and applying different types of measurement techniques that rely on solid-state NMR, they were able to complete the first structural model of amyloid-beta fibrils and address unsettled controversies about how they form.
“I think one of the important impacts of my lab’s work was helping to establish solid-state NMR as a really useful tool in structural biology,” says Dr. Tycko.

More From the IRP
Blog Post
IRP’s Peter Basser Elected to the National Academy of Engineering
In addition, his IRP team’s research on amyloid fibrils was important because it attracted the attention of other researchers. In particular, it led scientists to investigate whether variations in the molecular structure of amyloid aggregates might affect the progression or severity of Alzheimer’s disease. Although elderly people often have amyloid deposits in their brains, some of them experience significant cognitive impairment while others have barely any.
“We have some evidence that there are certain amyloid protein structures that form in specific variants of Alzheimer’s disease, but it's still very preliminary and we have a lot more measurements to do there,” says Dr. Tycko. “Whether variations in amyloid structures lead to variations in the severity or other aspects of neurodegenerative diseases is still an open question, and other labs are working on that as well.”
In addition to his work with solid-state NMR, Dr. Tycko is working to improve magnetic resonance imaging (MRI) methods so that MRI machines like the one pictured here can take snapshots of individual cells.
Dr. Tycko’s lab continues to find new applications for solid-state NMR and other technologies, such as cryogenic electron microscopy, a technique that visualizes the structures of samples at extremely low temperatures. For instance, Dr. Tycko worked with an NIDDK colleague, Reed Wickner, M.D., to apply solid-state NMR techniques to the study of prions, the proteins responsible for transmissible brain diseases like mad cow disease. They have also used these technologies to study amyloid plaques in the pancreas that are involved in type 2 diabetes and the protein lattices in HIV-1 virus particles.
At the same time, Dr. Tycko’s team continues to build and improve these analytic techniques, and his group is also experimenting with using solid-state NMR at lower temperatures than ever before, which allows them to literally take ‘freeze-frame’ measurements of proteins as they are assembled, folded, and interact with other proteins. Yet another project involves improving the resolution of magnetic resonance imaging to enable it to produce pictures not just of entire organs but also of the contents of individual cells.
“That is a good example of a very exploratory project,” explains Dr. Tycko. “I'm not really sure exactly what we're going to apply it to, but I just have this faith that if we can increase the resolution by an order of magnitude there must be an application. It's a big technological challenge and we’ve learned a lot by pursuing it.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
Related Blog Posts
This page was last updated on Wednesday, July 5, 2023
