Creating Cutting-Edge Cancer Vaccines
IRP Research Identifies a Tantalizing Target for Cancer Immunotherapy
Research led by IRP senior investigator Claudia Palena could lead to vaccines that help the immune system fight off cancer.
February 4 is World Cancer Day, a time to mark international efforts to prevent, detect, and treat cancer. Immunotherapy, one of the most significant advances in treating cancer, was pioneered here at NIH more than 30 years ago. Today, IRP senior investigator Claudia M. Palena, Ph.D., is pushing cancer immunotherapy forward with the discovery of a novel target for cancer vaccines.
Immunotherapy treatments for cancer, including cancer vaccines, harness the body’s own defenses to detect and kill any cells deemed foreign or abnormal, including tumor cells. Unlike vaccines against viruses, which prevent infection, cancer vaccines provoke the immune system to recognize and attack molecules found on tumors, including proteins that are more abundant on cancer cells and that may help them grow and spread through the body, a process called metastasis.
Dr. Palena’s research team discovered a protein that plays a key role in tumors’ ability to metastasize and become resistant to standard therapies like chemotherapy and radiation. The molecule, called T-box transcription factor brachyury, is a protein that was long thought to exist only on cells that are part of a developing embryo. However, Dr. Palena’s IRP team found abnormally high levels of brachyury in the cancer cells of adults with various types of carcinoma, a cancer that arises from the ‘epithelial’ cells that line most organs and the skin. The researchers found brachyury not just in the original tumor, but also in metastatic tumors that had spread from the original site to multiple other organs. By contrast, in normal, healthy tissue, the molecule was mostly absent.
“One of the challenges we faced when we started was that very little was known about brachyury,” says Dr. Palena. “We knew it played a role in embryonic development, but we didn’t know what role, if any, this molecule was playing in a cancer cell.”

Dr. Claudia Palena
The brachyury protein, it turns out, plays a key role in driving a process called the ‘epithelial-to-mesenchymal transition,’ or EMT.1 During early embryonic development, epithelial cells transform into mesenchymal cells, which have the capacity to further develop into multiple types of more mature cells, including connective tissue, blood vessels, and disease-fighting white blood cells. However, the EMT can also occur in carcinoma cells, granting them the ability to move throughout the body and become resistant to treatment. Dr. Palena’s team discovered that when the original, or 'primary,’ tumor that appeared in a patient’s body has high levels of brachyury, that patient is more likely to have a poor clinical outcome. This led her to theorize that targeting a protein like brachyury that regulates the EMT may be a valuable approach for a cancer vaccine.
“Our main drive and interest in studying brachyury was functional: by learning about its role, we became really interested in how it contributes to changes in tumor characteristics,” Dr. Palena says. “It’s different from other potential vaccine targets that are just over-abundant in cancer cells because brachyury also plays an important role in making tumors more aggressive.”
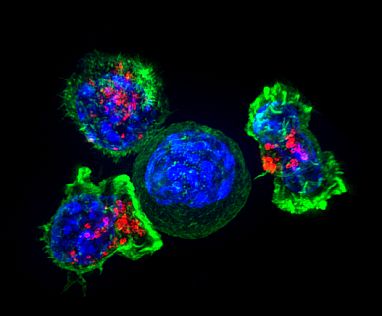
Killer T cells surround a cancer cell. These T cells attach to and spread over their target, then use special chemicals (red) to deliver a killing blow. Image credit: Alex Ritter, Jennifer Lippincott Schwartz, and Gillian Griffiths
This effort has already born fruit. Dr. Palena’s team showed that immune cells called T cells that have been isolated from a cancer patient’s blood can attack tumor cells that carry the brachyury protein.2 In addition, through collaborations with other groups of researchers both within and outside NIH, her team has contributed to the development of three brachyury-based cancer vaccines, which have already begun testing in Phase I and II clinical trials in patients with advanced cancers, including those with a rare type of tumor called a chordoma that appears in the spine or at the base of the skull.
Ultimately, Dr. Palena hopes that using cancer vaccines in combination with other cancer-fighting therapies could greatly improve outcomes for patients. To help bring that vision into reality, her laboratory is also studying molecules that are secreted in large amounts by cells that over-produce brachyury. Her team has already demonstrated that one of those molecules, interleukin-8, plays a role in making tumors more aggressive and might be a useful additional target to improve cancer immunotherapy.3
If her efforts are successful, Dr. Palena says, it will be in large part due to the opportunities NIH provides to work with different groups in the IRP, including the NIH Clinical Center and Tech Transfer Office, which has helped her team form collaborative relationships both within NIH and with outside biotech companies.
“The NIH is a highly collaborative environment, and this work is really a team science effort,” Dr. Palena says. “That was essential.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
References:
[1] Fernando RI, Litzinger M, Trono P, Hamilton DH, Schlom J, Palena C. The T-box transcription factor Brachyury promotes epithelial-mesenchymal transition in human tumor cells. J Clin Invest. 2010;120(2):533-44. doi: 10.1172/JCI38379.
[2] Palena C, Polev DE, Tsang KY, Fernando RI, Litzinger M, Krukovskaya LL, Baranova AV, Kozlov AP, Schlom J. The human T-box mesodermal transcription factor Brachyury is a candidate target for T-cell-mediated cancer immunotherapy. Clin Cancer Res. 2007 Apr 15;13(8):2471-8. doi: 10.1158/1078-0432.CCR-06-2353.
[3] Fernando RI, Castillo MD, Litzinger M, Hamilton DH, Palena C. IL-8 signaling plays a critical role in the epithelial-mesenchymal transition of human carcinoma cells. Cancer Res. 2011;71(15):5296-306. doi: 10.1158/0008-5472.CAN-11-0156.
Related Blog Posts
This page was last updated on Tuesday, May 23, 2023
