Synthetic Antibody Rallies Immune Cells Against Ovarian Cancer
Study Also Reveals Immunotherapy’s Target on Cancer Cells
A recent IRP study pitted an investigational immunotherapy against ovarian cancer and identified its binding target. The therapy uses Y-shaped molecules called antibodies (pictured above) that attach to cancer cells.
In the 1995 film The Usual Suspects, Kevin Spacey’s con man character famously remarks, “The greatest trick the Devil ever pulled was convincing the world he didn't exist.” The same could be said of cancer, which somehow persuades the body it is not a threat. Cutting-edge treatments called immunotherapies remove this façade and encourage the immune system to attack cancer cells. New IRP research in mice has demonstrated the promise of a new immunotherapy for treating ovarian cancer and identified a marker on cancer cells that could help clinicians identify patients who are most likely to benefit from the therapy.1
Because our body’s defenses typically ignore cancer cells, doctors rely on chemotherapy to destroy them instead. Unfortunately, chemotherapy also kills many heathy cells, and while it may be initially effective against a cancer, the disease often returns months or years after treatment has ceased. Moreover, when a cancer reappears, it may no longer be vulnerable to chemotherapy, as is often the case with ovarian cancer. Still other cancers, including ‘mucinous’ ovarian cancer, shrug off chemotherapy to start with, leaving patients with very limited treatment options.
Enter immunotherapy — in this case, a laboratory-produced antibody molecule called NEO-201 that was developed to combat colorectal cancer. Like the natural antibodies produced by our immune systems, NEO-201 binds to its target and draws in immune cells called natural killer cells to destroy it. In most people, the body does not produce antibodies that attach to cancer cells, making a synthetic antibody necessary to tell the immune system to attack them.
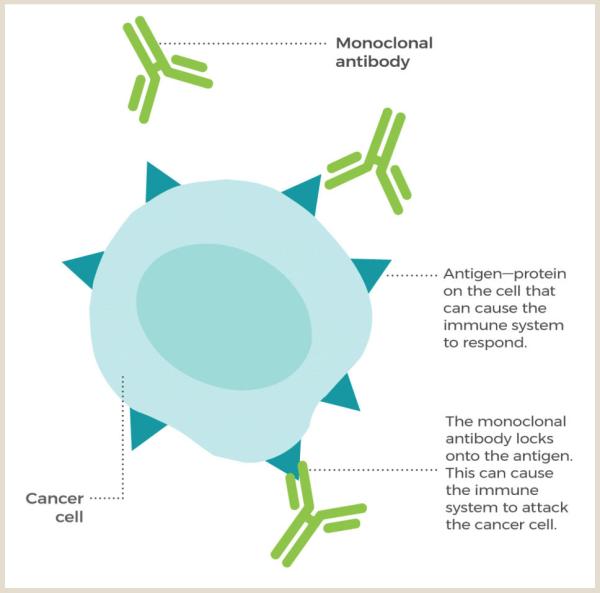
Image courtesy of the National Cancer Institute (NCI)
This diagram shows how antibody-based immunotherapies like NEO-201 trigger an immune response against cancer.
“If there was no antibody there, the natural killer cells would not really interact with the cancer cell because there would be no reason for them to recognize it as something to kill,” explains IRP senior investigator Christina Annunziata, M.D., Ph.D., the new study’s senior author.
While past research has shown that NEO-201 selectively binds to some pancreatic, breast, and lung cancers in addition to colorectal cancer,2 the specific molecule it attaches to on cancer cells remained a mystery until Dr. Annunziata’s team set out to identify it. First, the researchers examined which varieties of cancer NEO-201 can bind to, ultimately finding that it interacted with tumor cells from many colorectal and lung cancers and some breast and ovarian cancers, including chemotherapy-resistant mucinous ovarian cancer. Then, they examined NEO-201’s interactions with proteins from two cancer varieties they knew NEO-201 could bind to and one it could not. This analysis revealed the identity of two molecules that NEO-201 attaches to on cancer cells: carcinoembryonic antigen-related cell adhesion molecules 5 and 6, or CEACAM5 and CEACAM6 for short.
Further experiments confirmed the scientists’ discovery, as NEO-201 bound to isolated CEACAM5 and CEACAM6 but not two other CEACAM molecules. Moreover, when human cells that do not typically have CEACAM5 or CEACAM6 were manipulated to produce the two molecules, NEO-201’s ability to adhere to the cells dramatically increased. In contrast, reducing colorectal cancer cells’ ability to manufacture either or both of those CEACAM molecules reduced NEO-201’s ability to bind to the cells.

Dr. Christina Annunziata (left) talks with a patient. She is currently leading a clinical trial examining NEO-201’s safety in people with cancer.
“I think NEO-201 could provide a very targeted, precision medicine approach to treating cancers that have these specific molecules,” Dr. Annunziata says. “You might be able to select patients based on whether these altered CEACAM proteins are present on their cancer cells, but that’s something we would have to test in a future clinical trial.”
Dr. Annunziata’s study is also the first to test NEO-201’s effectiveness against ovarian cancer. As expected, like other immunotherapies, NEO-201 alone had no effect on isolated ovarian cancer cells, nor did natural killer cells on their own. However, ovarian cancer cells exposed to both NEO-201 and natural killer cells had a dramatically higher rate of cell death. Furthermore, in a mouse model designed to mimic ovarian cancer that had spread to other sites in the body, treatment with NEO-201 combined with an infusion of human immune cells dramatically extended the animals’ lives.
Dr. Annunziata is currently leading the first clinical trial ever to give NEO-201 to human patients. The study is assessing NEO-201’s safety in people with cancer, a necessary step on the way to trials testing its effectiveness. Eventually, NEO-201 could provide hope for patients with mucinous ovarian cancer and other cancers that fail to respond to chemotherapy.
“We really need new approaches for women with mucinous ovarian cancer,” Dr. Annunziata says. “I think this is a really exciting antibody approach for this unmet need.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
References:
[1] Evaluation of the Anti-Tumor Activity of the Humanized Monoclonal Antibody NEO-201 in Preclinical Models of Ovarian Cancer. Zeligs KP, Morelli MP, David JM, Neuman M, Hernandez L, Hewitt S, Ozaki M, Osei-Tutu A, Anderson D, Andresson T, Das S, Lack J, Abdelmaksoud A, Fantini M, Arlen PM, Tsang KY, Annunziata CM. Front Oncol. 2020 June 19;10:805. doi: 10.3389/fonc.2020.00805.
[2] Preclinical Characterization of a Novel Monoclonal Antibody NEO-201 for the Treatment of Human Carcinomas. Fantini M, David JM, Saric O, Dubeykovskiy A, Cui Y, Mavroukakis SA, Bristol A, Annunziata CM, Tsang KY, Arlen PM. Front Immunol. 2018 Jan 4;8:1899. doi: 10.3389/fimmu.2017.01899.
Related Blog Posts
This page was last updated on Tuesday, May 23, 2023
