Dying Tumor Cells Suppress Anti-Cancer Immune Response
IRP Study Points to Strategies to Stop Disease From Spreading
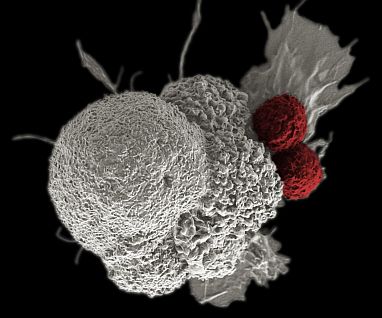
New IRP research points to new strategies that may help immune cells called T cells (red) more aggressively attack cancer cells (white) to prevent the disease from spreading through the body.
Ancient Greek myth describes how the hero Hercules battled the many-headed hydra, which regrew two heads every time Hercules cut one off. This frustrating fight against a seemingly invulnerable opponent would be an apt metaphor for treating cancer, in which tumor cells sometimes die in a particular way that actually helps their brethren multiply and spread to other parts of the body. In a study of that phenomenon using a mouse model of breast cancer, IRP researchers discovered that it occurs because that form of cell death suppresses the immune system’s response to the cancer,1 a finding that points to several potential ways to improve cancer therapy.
Our cells can die in several different ways, and the goal of many cancer treatments is to cause tumor cells to undergo a tightly regulated self-destruct process called apoptosis. However, perhaps because of low levels of nutrients and oxygen within a tumor, cancer cells are also constantly dying in another manner, known as necroptosis, without any outside intervention needed. While this might seem like a good thing, scientists have found that it can actually contribute to metastasis, the process in which a tumor spreads from its original location to distant sites in the body.
“One of the main reasons why cancer is such a bad disease is because of metastasis,” explains IRP senior investigator Zheng-Gang Liu, Ph.D., the new study’s senior author. “If you can detect the primary cancer in an early stage, it’s much easier to treat or even cure the cancer because you can take the cancer out with surgery, but when the cancer metastasizes and spreads to other parts of the body, you lose control of it. That’s why finding a way to control metastasis is a key factor for treating cancer.”
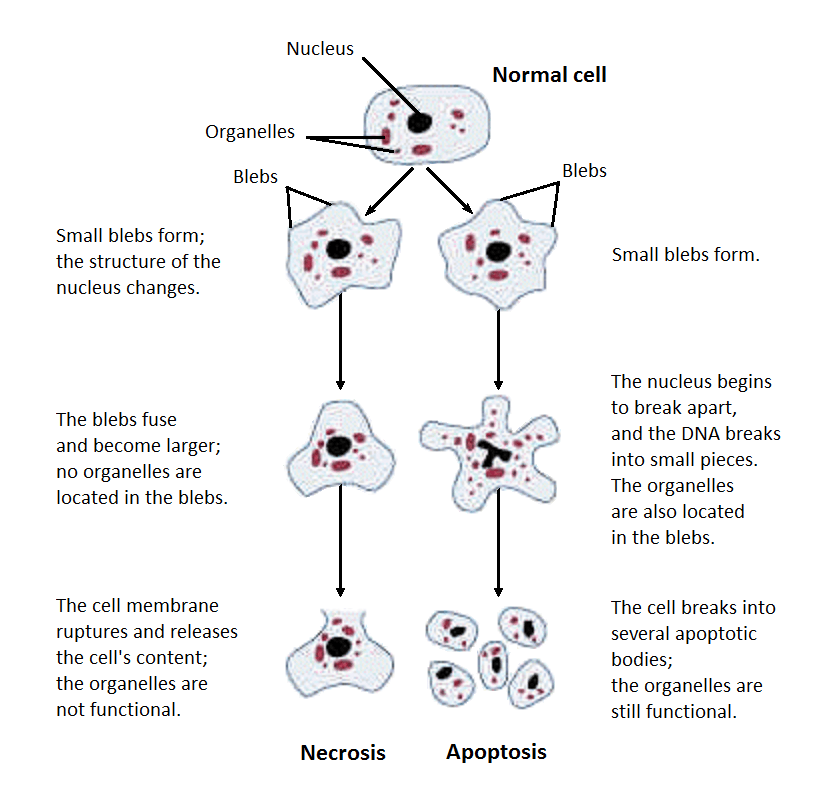
Necroptosis is a subtype of cell death within a broader category called necrosis. This diagram shows the differences in what happens to cells that undergo necrosis and apoptosis, which is a much more tightly controlled form of cell death.
In the new study, Dr. Liu’s team investigated the effects of blocking necroptosis by disabling the MLKL gene, which is vital for triggering it. In a mouse model that spontaneously develops breast cancer, the IRP researchers found that fewer cells were dying in the tumors of mice without the MLKL gene compared to those with an intact copy of the gene. Deleting the MLKL gene also significantly decreased the ability of the mice’s breast cancer to spread to their lungs. That observation was explained, at least in part, by the fact that the tumor cell death in the mice without MLKL was occurring via the tightly controlled process of apoptosis, rather than via necroptosis.
“Apoptosis is actually a tumor suppressor,” Dr. Liu says. “It normally controls tumor growth and metastasis. It’s the type of cell death we want to have in the tumor.”
The IRP study also looked at how blocking necroptosis affects immune cells called T cells in those mice. While deleting the MLKL gene did not change the number of T cells in the animals’ tumors, the T cells in the tumors of mice without MLKL were battling the cancer much more aggressively than those in the genetically normal mice. What’s more, when the mice without MLKL also lacked a specific type of T cell, known as CD8+ T cells, their cancers regained the ability to spread to their lungs, confirming the critical role T cells were playing in stopping metastasis in those mice.
“T cells are critical for anti-tumor immunity,” Dr. Lui explains. “When necroptosis is blocked, the activity of T cells is highly increased. As a result, the tumor’s growth and metastasis are greatly reduced.”

Dr. Zheng-Gang Liu
Finally, since necroptosis causes proteins on the surface of tumor cells to be jettisoned into the tumor’s surroundings but apoptosis does not, the IRP team examined how those ‘cell surface proteins’ interact with T cells. They first did this by treating genetically normal mice with a compound that inhibits molecules called ADAMs, which help proteins detach from a cell’s surface. Even though inhibiting ADAMs did not decrease necroptosis in the animals’ tumors, it still dramatically increased T cells’ anti-tumor activity and hindered the cancer’s spread to the mice’s lungs, just like deleting the MLKL gene did. The researchers also achieved the same effects in a third way: by stopping a particular cell surface protein from binding to its receptor on T cells, called the KLRG1 receptor. All together, these findings suggest that necroptosis promotes metastasis by releasing cell surface proteins that tamp down T cells’ response to cancer.
Dr. Liu’s team remains curious whether other aspects of necroptosis help tumors spread, including the influence of molecules inside tumor cells rather than on their surface. It is also still a mystery whether necroptosis in tumors caused by chemotherapy or radiation treatments might make cancer more likely to spread. As Dr. Liu’s team investigates those questions, it will simultaneously pursue the new and potentially life-saving strategies for combating cancer suggested by their study’s results.
“This study provides several therapeutic targets,” Dr. Liu says. “We could target the necroptosis with drugs that inhibit the proteins that induce it, or we could use drugs to inhibit the ADAMs that cause cell surface proteins to be released. Another target is KLRG1, since we showed neutralizing KLRG1 also inhibits metastasis. These findings could very well translate to human breast cancer, if not other cancers, to try to block metastasis.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
References:
[1] Liu Z, Choksi S, Kwon H, Jiao D, Liu C, Liu Z. Tumor necroptosis-mediated shedding of cell surface proteins promotes metastasis of breast cancer by suppressing anti-tumor immunity. Breast Cancer Res/ 2023 Jan 26;25(1):10. doi: 10.1186/s13058-023-01604-9.
Related Blog Posts
This page was last updated on Tuesday, May 23, 2023
