A Ray of Hope for a Rare and Deadly Skin Cancer
IRP Research Leads to First FDA-Approved Therapy for Merkel Cell Carcinoma

IRP senior investigator Dr. James Gulley (left), along with his IRP colleagues Dr. Jeffrey Schlom and Dr. Isaac Brownell, led IRP research that led to the first-ever FDA-approved treatment for a rare and deadly form of skin cancer.
May is Skin Cancer Awareness Month. Skin cancers are the most common cancer in the U.S., affecting as many as five million people every year. Yet the rarest of these cancers is also one of the deadliest. Merkel cell carcinoma affects about 3,000 Americans each year, and until recently a lack of effective treatments meant only half of patients survived five years or longer after diagnosis. The median survival was nine months.
This bleak outlook changed radically in 2017 with the US Food and Drug Administration (FDA) approval of a new immunotherapy drug called avelumab. Developed through a collaboration between IRP researchers and the pharmaceutical company EMD Serono, Inc., and marketed as Bavencio, avelumab was the first treatment approved specifically for Merkel cell carcinoma.
“Avelumab changed the way we treat this cancer and it changed the prognosis of this cancer,” says IRP investigator Isaac Brownell, M.D., Ph.D., who co-led the preclinical research on avelumab at the NIH along with IRP senior investigators James Gulley, M.D., Ph.D., and Jeffrey Schlom, Ph.D.
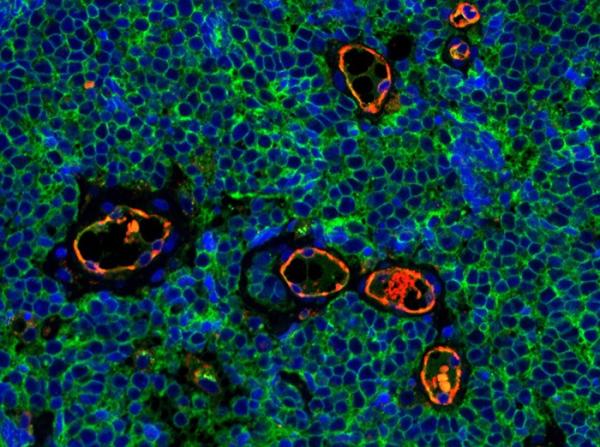
Immunofluorescent staining of Merkel cell carcinoma tumor tissue.
Although the skin tumors caused by Merkel cell carcinoma are usually painless, they tend to grow and spread very quickly. If caught very early, the disease can be treated, usually with surgery and radiation. Once it begins to spread through the body, however, it becomes very difficult to treat. Chemotherapy can shrink the tumors, but they often return within months. People over age 70, especially men and individuals with weakened immune systems, are at highest risk for the cancer.
Avelumab is a member of a class of immunotherapies known as ‘checkpoint inhibitors.’ To function properly — and not attack normal cells — the immune system is controlled by a protein on each immune cell called a ‘checkpoint.’ These are basically on/off switches that power up an immune attack when a target that shouldn’t be in the body, such as an infection or cancer cell, turns them on. Many of the body’s normal cells are marked with a protein called programmed death-ligand 1 (PD-L1), which keeps the immune checkpoint in the ‘off’ position to prevent immune cells from attacking healthy cells. However, some forms of cancer, including Merkel cell carcinoma, also have PD-L1, thereby preventing the immune system’s T cells from launching an assault against them.
“The T cell may be specific to that tumor, may recognize that tumor, may be able to kill that tumor, but it’s prevented from doing so because the PD-L1 marker is present” Dr. Gulley explains. “Avelumab works like putting a bag over a stop sign — it hides the PD-L1 from the T cells, so they just go ahead and do their work.”
More From the IRP
Blog
Synthetic Antibody Rallies Immune Cells Against Ovarian Cancer
Dr. Gulley and Dr. Schlom began working on avelumab in 2013 when the chief medical officer at EMD Serono invited Dr. Gulley to collaborate on a promising agent in their product pipeline.
“It was a perfect storm in terms of the partnership between the private sector and the Intramural Research Program,” Dr. Schlom says. “A former staff scientist who had worked in my lab went on to become head of immunology at EMD Serono. Since she had worked in our laboratory, we knew each other very well and we could work together extremely well.”
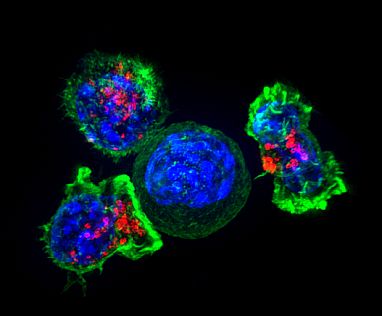
Image credit: Alex Ritter, Jennifer Lippincott Schwartz, and Gillian Griffiths, NIH
Killer T cells surround and attack a cancer cell.
The initial safety and efficacy trials of avelumab focused on treating people with a variety of cancers that the researchers believed would respond to immunotherapy. The IRP researchers and their collaborators at EMD Serono conducted what the FDA calls a ‘seamless trial,’ which is intended to accelerate the development and approval of cancer treatments without compromising patient safety by compressing the first three phases of clinical trials into a single continuous trial that gradually increases patient numbers and the dosage of the treatment they receive. As a result, they were able to enroll more than 1,700 cancer patients in the study over a short period of time.
During the trial period, Dr. Brownell, a dermatologist at the NIH Clinical Center, approached Dr. Gulley for help designing a study specifically for Merkel cell carcinoma. This sparked the idea to also test avelumab in 88 patients with an advanced form of the cancer. Even though the enrolled patients had advanced disease that had not responded to chemotherapy, 33 percent saw their tumors shrink, and many of those patients saw improvements for six months or longer. In fact, the effects were evident at the first scan after only six weeks of taking avelumab. Today, some of the patients treated in that 2013 trial are still alive.
“We have not been using this approach long enough to know what the median survival is now, but it’s greatly extended,” Dr. Brownell says.

Dr. Jeffrey Schlom (left) and Dr. Isaac Brownell (right)
The rapid progress of the seamless trial, combined with the dramatic results of the study focused on Merkel cell carcinoma, led to an unusually rapid approval by the FDA to use avelumab to treat that type of cancer. Less than four and a half years after the first patient began participating in the seamless trial, the FDA gave its stamp of approval. A year later, in recognition of their achievement, the IRP researchers who worked on these trials received the 2018 Hubert H. Humphrey Award for Service to America.
While avelumab is game-changing for Merkel cell carcinoma, it is having an impact on other cancers as well. The FDA has already approved avelumab for treating two types of bladder cancer and for treating renal cell carcinoma in combination with another drug. In addition, the IRP researchers who worked on avelumab are now collaborating with the NIH’s National Center for Advancing Translational Science (NCATS) to identify drugs that could work well in combination with avelumab.
“Even though Merkel cell is a rare disease, our work had an important impact,” Dr. Gulley says. “Thanks to the ability to pull in patients from all over the U.S., I would argue that the NIH’s intramural program is a great place to study rare diseases.”
References
[1] Avelumab for metastatic or locally advanced previously treated solid tumours (JAVELIN Solid Tumor): a phase 1a, multicohort, dose-escalation trial. Heery CR, O'Sullivan-Coyne G, Madan RA, Cordes L, Rajan A, Rauckhorst M, Lamping E, Oyelakin I, Marté JL, Lepone LM, Donahue RN, Grenga I, Cuillerot JM, Neuteboom B, Heydebreck AV, Chin K, Schlom J, Gulley JL. Lancet Oncol. 2017; 18(5):587-598.
[2] Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Kaufman HL, Russell J, Hamid O, Bhatia S, Terheyden P, D'Angelo SP, Shih KC, Lebbé C, Linette GP, Milella M, Brownell I, Lewis KD, Lorch JH, Chin K, Mahnke L, von Heydebreck A, Cuillerot JM, Nghiem P. Lancet Oncol. 2016; 17(10):1374-1385.
[3] Antibody-Dependent Cellular Cytotoxicity Activity of a Novel Anti-PD-L1 Antibody Avelumab (MSB0010718C) on Human Tumor Cells. Boyerinas B, Jochems C, Fantini M, Heery CR, Gulley JL, Tsang KY, Schlom J. Cancer Immunol Res. 2015; 3(10):1148-57.
Related Blog Posts
This page was last updated on Tuesday, May 23, 2023
