IRP’s Giorgio Trinchieri Elected to National Academy of Sciences
IRP Scientist Has Found New Ways to Wield the Immune System Against Cancer

Dr. Giorgio Trinchieri has spent his career exploring the immune system and how it might be better leveraged to combat cancer.
Over the past twenty or so years, we’ve seen a sea change in many cancer therapies resulting from advances in immunotherapy. Rather than trying to poison cancer cells with chemotherapy or rip apart their DNA with radiation, these treatments help our own bodies attack the disease. As a result, we’ve seen a remarkable reduction in cancer deaths from many types of tumors.
NIH Distinguished Investigator Giorgio Trinchieri, M.D., has long stood at the forefront of these discoveries. This year, he was elected to the National Academy of Sciences (NAS) for his achievements in identifying the mechanisms regulating the activity of certain classes of immune cells and how the environment around a tumor interacts with the immune system to suppress an immune response to the disease. This includes searching for ways to expand the benefits of immunotherapy to more patients.
“Unfortunately, not all types of tumors respond to cancer immunotherapy,” says Dr. Trinchieri. “It’s a good number that do, depending on the type of cancer — from 20 to 30 percent, or even up to 50 percent, but it’s definitely not 100 percent.”
Dr. Trinchieri has spent his entire career studying the immune system, so when he came to NIH in 2006, it was a natural move to apply his expertise to figuring out how to increase the number of patients who respond to cancer immunotherapy.
“My background is really in immunology and my interest has always been how the body regulates our ability to mount a new response either against bugs or against cancer,” says Dr. Trinchieri. “In the last 15 to 20 years, we’ve recognized that the strength of our immunity is not only affected by pathogens, but by the microorganisms that naturally live within our bodies.”
These benign, native microorganisms, called the microbiome, are very important for our health in general, including how the immune system behaves. For instance, the composition of a person’s microbiome affects his or her body’s response to a vaccine or an infection.

Dr. Trinchieri’s research suggests that the benign microbes that live peacefully in our bodies influence the effectiveness of cancer treatments.
In 2013, Dr. Trinchieri’s research team at NIH, along with a separate research group in France, were among the first research teams to consider that if the microbiome can influence the immune system’s response to infectious diseases, it might also play a role in how the immune system deals with cancer during therapy. To explore that question, they conducted two experiments using ‘germ-free’ mouse models, in which the animals’ microbiomes had been eradicated.
In some of the animals, Dr. Trinchieri and his colleagues injected DNA into tumor cells to stimulate an immune reaction, while in others they treated the tumor cells with a common type of chemotherapy. These mice responded poorly to either therapy, suggesting that the body’s native microbes have a substantial influence on whether a cancer treatment works. The group in France tried similar experiments with other types of chemotherapy drugs and had the same results.1,2
These findings got people interested in looking at how the microbiome might influence the effectiveness of the most common type of immunotherapy, called an immune-checkpoint inhibitor. This treatment strategy stops tumors from sending ‘off’ signals to immune cells called T cells, thereby enabling the cells to attack cancer cells. At that point in time, there was some evidence suggesting the microbiome could influence the effectiveness of immune-checkpoint inhibitors, “but the problem was many of these studies showed a variety of mechanisms and a variety of bacteria at play, which caused some controversy in the field,” Dr. Trinchieri says.
Consequently, Dr. Trinchieri’s group decided to collaborate with clinical investigators at the University of Pittsburgh on a study of cancer patients rather than animal models, with the aim of identifying which bacteria were key to the success or failure of the patients' treatments. Specifically, they analyzed the types of gut bacteria in cancer patients and compared the gut microbiomes of patients who responded to cancer immunotherapy and those who didn’t. Next, they transplanted healthy gut bacteria from the responsive patients into the non-responsive ones to see if they could turn the latter into the former. The results strongly suggested that, like in the mice, having a certain composition of bacteria in the gut aided the body’s immune system in fighting cancer.3
“Even though it was a small trial of 15 patients, 40 percent of those who had the microorganism transfer had a good response to immunotherapy,” Dr. Trinchieri says.
“I think we contributed to clarifying that although the results of microbiome studies may look very different, there were common mechanisms shared across them and the microbiome can be targeted therapeutically to improve patients’ response to therapy,” he adds.
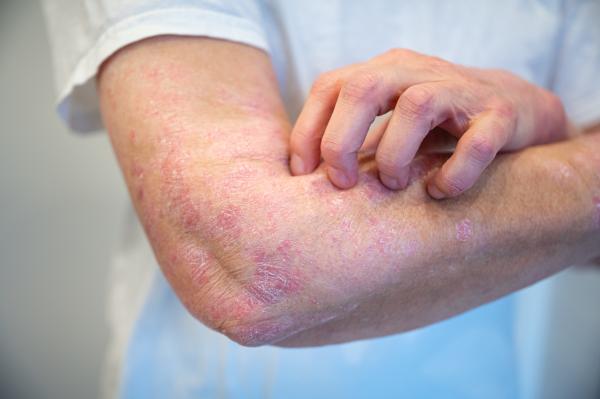
Dr. Trinchieri’s research on the immune system has also had applications to several conditions caused by an over-reactive immune system, such as the irritating skin ailment psoriasis.
Along with his more recent research at NIH, Dr. Trinchieri believes his election to the NAS was partly inspired by his discovery, in 1989, of a molecule called interleukin-12 (IL-12) while he was working at the Wistar Institute in Philadelphia.4 IL-12 is one of several types of ‘cytokines,’ small proteins that play a crucial role in the immune system and the development of blood and immune cells. His team at Wistar ultimately created antibody molecules capable of inhibiting IL-12 for use in their studies, which were later leveraged as the basis for treatments for psoriasis, psoriatic arthritis, and inflammatory bowel disease.
Even though Dr. Trinchieri has an M.D., his work has long focused exclusively on research rather than working directly with patients; in fact, he chose to go to medical school primarily because he wanted to do research. However, despite not seeing patients himself, he views his work as a kindred effort to improve their health.
“The way to approach human health, obviously, is to apply what we know to improve therapy and disease prevention, but we can't really do that unless we understand the context in which immunotherapy is operating,” Dr. Trinchieri says. “Basic science research remains essential when we are trying to do that.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
References:
[1] Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, Molina DA, Salcedo R, Back T, Cramer S, Dai RM, Kiu H, Cardone M, Naik S, Patri AK, Wang E, Marincola FM, Frank KM, Belkaid Y, Trinchieri G, Goldszmid RS. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013 Nov 22;342(6161):967-70. doi: 10.1126/science.1240527.
[2] Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillère R, Hannani D, Enot DP, Pfirschke C, Engblom C, Pittet MJ, Schlitzer A, Ginhoux F, Apetoh L, Chachaty E, Woerther PL, Eberl G, Bérard M, Ecobichon C, Clermont D, Bizet C, Gaboriau-Routhiau V, Cerf-Bensussan N, Opolon P, Yessaad N, Vivier E, Ryffel B, Elson CO, Doré J, Kroemer G, Lepage P, Boneca IG, Ghiringhelli F, Zitvogel L. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science. 2013 Nov 22;342(6161):971-6. doi: 10.1126/science.1240537.
[3] Davar D, Dzutsev AK, McCulloch JA, Rodrigues RR, Chauvin JM, Morrison RM, Deblasio RN, Menna C, Ding Q, Pagliano O, Zidi B, Zhang S, Badger JH, Vetizou M, Cole AM, Fernandes MR, Prescott S, Costa RGF, Balaji AK, Morgun A, Vujkovic-Cvijin I, Wang H, Borhani AA, Schwartz MB, Dubner HM, Ernst SJ, Rose A, Najjar YG, Belkaid Y, Kirkwood JM, Trinchieri G, Zarour HM. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science. 2021 Feb 5;371(6529):595-602. doi: 10.1126/science.abf3363.
[4] Kobayashi M, Fitz L, Ryan M, Hewick RM, Clark SC, Chan S, Loudon R, Sherman F, Perussia B, Trinchieri G. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989 Sep 1;170(3):827-45. doi: 10.1084/jem.170.3.827.
Related Blog Posts
This page was last updated on Monday, August 12, 2024
