IRP Research Yields Life-Changing Treatments
Highlighting Drugs and Vaccines Stemming from NIH Discoveries
More than two dozen FDA-approved medical interventions that entered the market over the past five decades relied on IRP discoveries.
On May 3, more than six decades of IRP research culminated in the U.S. Food and Drug Administration (FDA) approving the world’s first vaccine for respiratory syncytial virus (RSV), a disease that puts tens of thousands of Americans in the hospital each year and kills thousands. While the new vaccine, called Arexy, has been getting all the headlines recently, it is only the latest example of a slew of FDA-approved medications and vaccines that might never have existed without the tireless efforts of scientists at NIH.
Indeed, a recently published study led by Mark Rohrbaugh, Ph.D., J.D., a special advisor in the NIH Office of Science Policy, found that inventions developed at NIH have contributed to more FDA-approved products than those created at any other nonprofit research institution in the world over the past five decades.1 NIH tops the study’s list with 27 FDA-approved products, six more than the study attributed to the combined efforts of all the schools in the University of California system.
In light of these two events, let’s take a look at some of those products to celebrate how IRP research has left the lab to change lives on a wide scale. The following list is by no means comprehensive, but it provides a taste of how important that work is for developing life-saving medical interventions.
IRP research led to the development of a vaccine for the human papillomavirus (HPV), which will help prevent countless cases of cervical and other cancers.
IRP senior investigators Douglas Lowy, M.D., and John Schiller, Ph.D., have dedicated their careers to studying the human papillomavirus (HPV), a cancer-causing infection that is also the world’s most common sexually-transmitted disease. The virus is thought to cause more than 90 percent of cervical and anal cancers, and according to the U.S. Centers for Disease Control and Prevention (CDC), more than 47,000 new HPV-related cancers occur in the U.S. each year.
Those numbers are poised to drop significantly in the coming years, however, thanks in large part to researchers led by Drs. Lowy and Schiller. The IRP scientists discovered a way to produce ‘virus-like particles’ (VLPs) that mimic HPV but don’t cause disease, providing a safe and effective way to teach the immune system to fight off the infection before it can take root and contribute to cancer. This key advance led to the development and 2006 FDA-approval of Gardasil, the first commercially available vaccine against four forms of the virus, including the two most deadly strains. A new version that protects against more strains of the virus and can be given to individuals in a larger age range, Gardasil 9, received FDA approval in 2014. Another vaccine based on the same VLP technology, Cervarix, was approved by the FDA in 2009 to protect against two strains of HIV that cause about 70 percent of cervical cancers. Though it is no longer sold in the U.S., Cervarix continues to be used in the European Union. Today, the CDC recommends that all boys and girls receive the HPV vaccine at 11 or 12 years of age and that anyone up to age 26 who has not yet been vaccinated receive the vaccine.
A Treatment for a Painful Side Effect of Cancer Therapy
In 1989, a trio of scientists working at NIH — Jeffrey Rubin, M.D., Ph.D., Paul Finch, Ph.D., and Stuart A. Aaronson, M.D. — discovered and purified a substance called keratinocyte growth factor (KGF). Subsequent studies found that KGF could help painful, infection-prone ulcers in the mouth heal more quickly by stimulating the growth of new cells. This proved to be a boon for cancer patients, since painful mouth sores are a common side effect of certain cancer therapies.
NIH soon entered into a partnership with biotechnology company Amgen to create a therapy based on KGF called Kepivance, which received FDA approval in 2004. Today, the medication is given to thousands of cancer patients each year to combat the painful mouth sores that result from their treatment.
A Synthetic Substitute for a Crucial Hormone
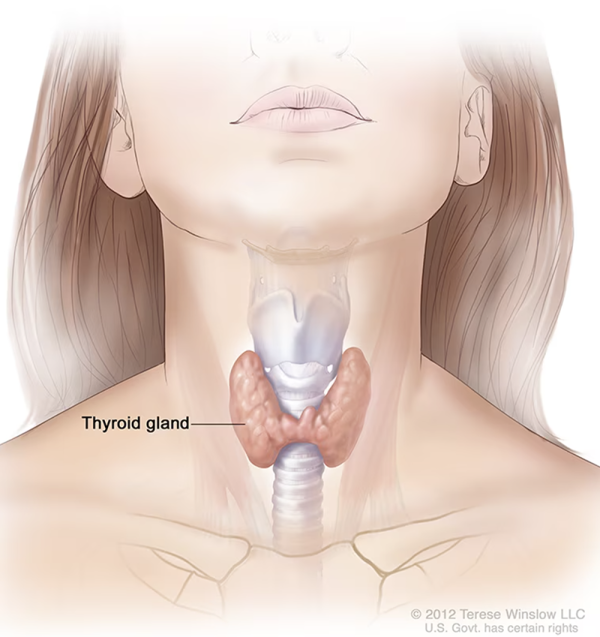
The thyroid, located in the neck, makes several crucial hormones when it is activated by thyroid-stimulating hormone (TSH).
Incredibly, scientists can achieve in a matter of years what nature took millennia to accomplish. This is the case with Thyrogen, a lab-produced version of thyroid stimulating hormone (TSH) developed with the help of IRP scientists. TSH, produced by the pituitary gland, stimulates the thyroid gland to release important hormones, but people whose thyroid has been removed to combat thyroid cancer receive artificial replacements for those thyroid hormones, so their bodies don’t need to produce as much TSH.
A lack of TSH isn’t a problem for these patients most of the time, but it poses an obstacle when doctors want to make sure their cancers haven’t returned months or years later. This is because the tests that detect thyroid cancer cells in the body require a certain amount of TSH, as do certain treatments for the disease. Consequently, for those tests and treatments to work, patients would need to temporarily stop taking synthetic thyroid hormones, which prompts their bodies to produce more TSH but causes significant side effects due to the loss of those hormones.
Fortunately, that often isn’t necessary these days thanks to the IRP discoveries that led to Thyrogen, which received FDA approval in 1998. Because Thyrogen acts like TSH in the body, it allows tests and treatments for thyroid cancer to work even while patients keep taking synthetic thyroid hormone replacements to keep their bodies running normally. In this way, with the help of a medication created largely through NIH research, patients can maintain a much higher quality of life while undergoing treatment or monitoring for thyroid cancer.
Disco-Era Discovery Pays Dividends Decades Later
In 1976, IRP researchers led by Robert Gallo, M.D., discovered a molecule called interleukin-2 that certain immune cells make in order to rev up the growth and activity of other immune cells. To exert its effects, interleukin-2 must bind to a ‘receptor’ on immune cells like a key fits into a lock. Consequently, blocking that receptor prevents interleukin-2 from doing its job — an effect that eventually turned out to have multiple important clinical applications.
For example, researchers led by IRP senior investigator Thomas Waldmann, M.D., who passed away in 2021, demonstrated how a particular type of protein called an ‘antibody’ could be used to block the IL-2 receptor in order to treat certain cancers and autoimmune diseases, which occur when the immune system becomes hyper-active. That work set the foundation for the 1997 FDA approval of Zenapax for people who have received a kidney transplant. When a patient receives an organ transplant, his or her body often attacks it, a phenomenon known as rejection. Zenapax suppresses the immune system’s response to the new kidney, thereby preventing rejection and its potentially life-threatening consequences.
Although Zenapax’s manufacturer, pharmaceutical company Roche, stopped producing it in 2009 due to shrinking market demand and the availability of alternative treatments, IRP research on IL-2 has provided the foundation for other therapies as well. Nearly two decades after the discovery of interleukin-2, in the 1990s, a synthetic version of the molecule marketed under the name Proleukin would receive FDA approval as a treatment for two types of cancer — the skin cancer melanoma and the kidney cancer renal cell carcinoma — when the disease has become ‘metastatic,’ meaning it has spread through the body from its site of origin. Proleukin works by revving up the activity of immune cells so that they attack the cancer. Other so-called ‘immunotherapies’ based on studies of interleukin-2 are also being developed at NIH and elsewhere, making it likely that the initial discovery by Dr. Waldmann’s IRP team will continue bearing fruit well into the future.
Sometimes the immune system itself is the source of cancer, as is the case with B-cell non-Hodgkin’s lymphoma, which occurs when the body makes abnormal immune cells called B cells. In 2002, the FDA approved Zevalin as a treatment for this disease, which affects approximately 50,000 Americans each year.
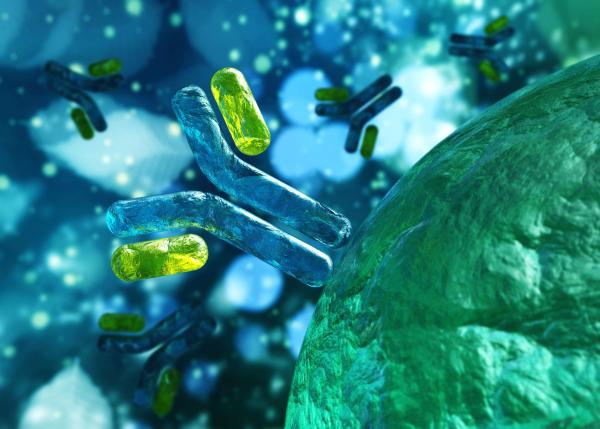
The drug Zevalin, a treatment for B-cell non-Hodgkin’s lymphoma, is made up of a synthetic antibody that binds to cancer cells and a radioactive atom attached to the antibody.
Zevalin has two key components. The first is a lab-made antibody that binds to a particular protein found on the surface of B cells, which causes the immune system to destroy those cells. The second is a radioactive atom attached to that antibody, which damages the cancerous cell that the antibody binds to and any cells nearby. Zevalin was the first-ever FDA-approved cancer treatment that relies on this dual immunotherapy-radiation strategy, and its development hinged on IRP research into a method for delivering the radioactive atom specifically to the site of the cancer to prevent it from harming other parts of the body.
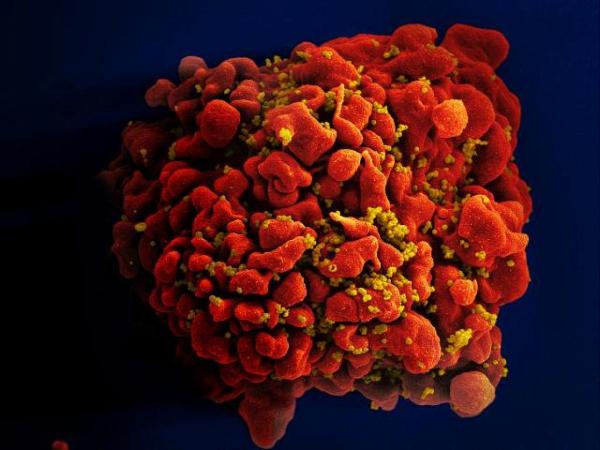
Scanning electron micrograph of an HIV-infected T cell. HIV causes AIDS by killing these cells, thereby crippling the immune system.
Long before COVID-19 came on the scene, NIH scientists made major contributions to the war against another infectious epidemic: AIDS. Not only were IRP researchers among the first to discover that the virus now known as HIV causes AIDS, but they also played a key role in the creation of the first HIV treatments.
Among these was a molecule called didanosine, which was initially synthesized by scientists at Brigham Young University in 1964. A group of scientists at NIH later conducted experiments that demonstrated didanosine’s ability to inhibit HIV’s replication in test tubes, as well as initial clinical trials that showed its effectiveness at combating the virus in patients. After receiving a patent for the treatment based on these results, NIH licensed the patent to the pharmaceutical company Bristol-Myers Squibb, which began producing and selling didanosine under the name Videx. Videx received FDA approval as a treatment for HIV/AIDS in 1991 and has since become a crucial component of the medication combo known as highly active antiretroviral therapy (HAART), the standard treatment for suppressing the virus in patients with HIV.
These examples provide just a small sample of the ripple effects IRP research has had across the medical landscape. NIH scientists have also played pivotal roles in the creation of a treatment for the cancer multiple myeloma, vaccines for diseases including rotavirus and hepatitis A, and many more important medical interventions. With this track record, there’s no doubt that the many discoveries being made at NIH today will go on to help save countless lives in the future.
References:
[1] Stevens AJ, Benson DE, Dodson SE, Jensen HH, Rohrbaugh ML. Role of global public sector research in discovering new drugs and vaccines. J Technol Transf. 2023 Apr 27. doi: https://doi.org/10.1007/s10961-023-10007-z.
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
Related Blog Posts
This page was last updated on Wednesday, July 5, 2023
