IRP’s John T. Schiller Elected to National Academy of Sciences
NIH Scientist’s Decoy Virus Revolutionizes Cervical Cancer Prevention

IRP researcher John T. Schiller was elected to the National Academy of Sciences in 2020 for his crucial contributions to the development of vaccines for human papillomaviruses (HPV), which can cause cervical cancer.
The National Academy of Sciences (NAS), established in 1863, is comprised of the United States’ most distinguished scientific scholars, including nearly 500 Nobel Prize winners. Members of the NAS are elected by their peers and entrusted with the responsibility of providing independent, objective advice on national matters related to science and technology in an effort to advance innovations in the United States.
IRP senior investigator John T. Schiller, Ph.D., was elected to the NAS in 2020 in recognition of a career that has produced numerous discoveries about human papillomaviruses (HPV), sexually transmitted infections that cause genital warts and are responsible for most cases of cervical cancer. His decades-long partnership with fellow IRP senior investigator Douglas R. Lowy, M.D., who was elected to the NAS in 2009, has yielded a deeper understanding of how HPV infects and damages cells and led to the creation of the first vaccines to prevent HPV infection.
“It’s really the best you can hope to achieve because it’s recognition by your peers,” Dr. Schiller says of the honor. “It’s not just for one success. It’s for your whole body of work.”
Dr. Schiller joined Dr. Lowy’s lab at the NIH’s National Cancer Institute (NCI) as a postdoctoral fellow in 1983 after receiving his doctorate in microbiology from the University of Washington. He has been at the NCI ever since, where he runs a section in the Laboratory of Cellular Oncology. His central research, including the work that led to the HPV vaccine, focuses on understanding the molecular mechanics of how HPV infects cells and triggers the changes that lead to cancer. His IRP team is also finding ways to apply the insights it learns from this research to the development of preventive vaccines and therapies for cervical and other cancers, as well as other conditions such as heart disease.
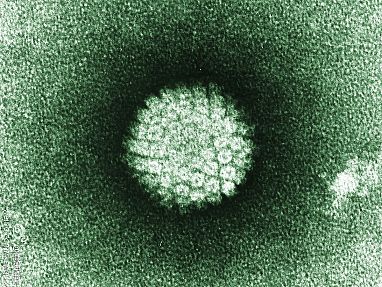
Image credit: Laboratory of Tumor Virus Biology, NIH National Cancer Institute
HPV up close.
Half a million women are diagnosed with cervical cancer worldwide each year, and more than 250,000 die annually from the disease. In the U.S., where more effective screening programs have reduced cases significantly, there are still nearly 14,000 cases reported each year and over 4,000 deaths. When the link between HPV and cervical cancer was discovered in 1983, it became clear that a vaccine could make a huge difference in women’s health. HPV’s genome contains cancer-promoting genes called oncogenes, and if HPV infection persists, the virus’s oncogenes can alter normal cells over time, transforming them into cancerous ones. Although the body is usually adept at clearing HPV infection long before it progresses to cancer, scientists hoped that a vaccine would curb rates of cervical cancer by preventing infection in the first place.
However, creating a vaccine for HPV posed unique challenges. Most vaccines are made from crippled versions of the viruses they protect against. These vaccines have nearly wiped out many diseases, such as polio and measles, by training the immune system to combat viruses without causing an actual infection. However, even in a weakened state, HPV and its cancer-causing oncogenes are too dangerous to introduce into the body, and previous attempts at creating a vaccine had failed. A different kind of strategy for triggering an immune reaction was needed.

More From the IRP
Podcast
Dr. Ira Pastan and Dr. Michael Gottesman — Cancer Immunotoxins and Multidrug Resistance
The answer to this conundrum, developed by Dr. Schiller’s and Dr. Lowy’s research team in the early 1990s, was to create a vaccine that contained only the outer shell of the virus. The IRP scientists used the protein particles from HPV’s surface to do this in the hopes that they would re-form in the same shape as the full virus’s outer shell. The experiment succeeded, resulting in a decoy called a virus-like particle (VLP) that mimicked HPV’s outer appearance but lacked its harmful genetic guts.
“They’re just a glob of protein,” Dr. Schiller says of the VLPs. Yet the results were dramatic. “This is an amazingly effective vaccine that nobody thought would work as well as it did.”
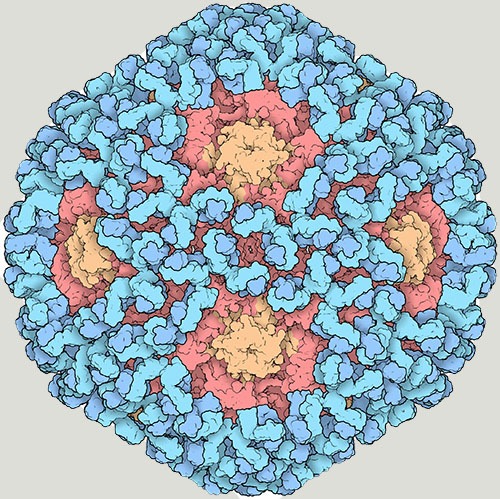
Image credit: David S. Goodsell, Research Collaboratory for Structural Bioinformatics Protein Data Bank
This cryo-electron microscope image shows the outer shell of HPV, known as the capsid (red and orange), covered by virus-specific antibodies (blue).
After this critical advancement, Dr. Schiller and Dr. Lowy continued to study their VLPs to understand why the vaccine worked so well. Two characteristics ultimately stood out. First, the immune response the vaccine generated was huge, with the VLPs triggering a veritable onslaught of antibody molecules that bind to HPV and thereby prevent infection. Second, the two scientists noted that because HPV infection is an unusually slow process, taking hours to days, the vaccine-induced antibodies have a long time to seek out the virus, bind to it, and block it. Together, those two factors meant their HPV vaccine had plenty of time to do its work and provided the body with more than enough ammunition to inactivate the virus.
“We hope that some of these things that we’ve learned can be used to develop future generations of vaccines,” says Dr. Schiller.
In fact, that work has already begun. Dr. Schiller’s lab is looking at a variety of uses for VLPs to combat other cancers, as well as other classes of disease. For example, he is collaborating with other IRP investigators in an attempt to use VLPs to create a vaccine that lowers the risk of heart disease by causing the immune system to produce antibodies that bind to and inhibit a protein called PCSK9, which regulates the amount of artery-clogging cholesterol in the blood. This vaccine could be administered at a lower cost than other treatments designed to target PCSK9. Another promising application of Dr. Schiller’s research is the creation of cancer-fighting VLPs. These VLPs selectively attach to cancer cells and contain a dye activated by infrared light. When the VLPs encounter cancerous tissue, doctors would zap the area with an infrared laser to trigger the release of molecules called ‘free radicals’ that can destroy cancer cells.

Image credit: The Albert and Mary Lasker Foundation
Dr. Schiller (left) and his long-time collaborator, Dr. Douglas R. Lowy (right), received the 2017 Lasker-DeBakey Clinical Medical Research Award for their work on HPV vaccines. The Lasker award is the country’s most prestigious biomedical research prize.
“We can use these particles like guided missiles to deliver cell-killing agents specifically to the cancer cells,” explains Dr. Schiller.
Dr. Schiller’s lab is currently collaborating with a company called Aura Biosciences to further hone that approach, and his team is also working with researchers at Oxford University on a treatment that uses a different dye that can be activated using high-frequency sound waves known as ultrasound, which can penetrate deeper into the body than infrared light. That approach could be particularly useful in settings with relatively few healthcare resources, since ultrasound is a relatively low-cost and low-maintenance technology.
Over his 35 years at the NIH, Dr. Schiller has watched his field expand from basic science to real-world implementation in public health, and his own point of view has widened as well. He hopes more companies and institutions will proactively put greater focus at the outset on how interventions can serve low-resource communities, rather than adapting treatments and technologies to these settings later.
“It really does broaden your perspective to think about these treatments from a global public health point of view and how they can be implemented in a low-resource setting,” he says. “What we do in the lab may be complex, but the goal is to develop interventions that are inexpensive and simple to deliver.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
Related Blog Posts
This page was last updated on Monday, January 29, 2024
