Overzealous Immune Cells Hamper Healing
Study Points to Treatment Targets for Impaired Healing Due to Diabetes
Image credit: Marco Gutierrez, Indiana University School of Medicine
IRP researchers have discovered important clues as to why certain foot injuries often fail to heal in patients with diabetes.
Whether we’ve nicked a finger while chopping vegetables or wiped out riding a skateboard, we tend to take for granted that our injuries will eventually mend themselves. However, for a type of wound that often plagues patients with diabetes, healing is no sure thing. IRP researchers recently identified why certain immune cells shift from helpful healers into saboteurs in those injuries.1
As many as a quarter of diabetic patients will develop a wound called a diabetic foot ulcer (DFU) sometime in their lives. These wounds occur due to a combination of factors related to the chronically elevated blood sugar levels of people with diabetes. Unfortunately, unlike typical injuries in people without diabetes, DFUs often stubbornly refuse to heal, frequently getting infected and sometimes requiring doctors to amputate the affected limb. What’s more, the few FDA-approved drugs available to treat DFUs fail to help more than half of patients.
“Those were approved over 20 years ago, and there really hasn’t been anything new since,” says Andrew Sawaya, Ph.D., who led the new study as a postdoctoral fellow in the lab of IRP senior investigator Maria Morasso, Ph.D., before taking a position at the University of Miami’s Miller School of Medicine.

Dr. Maria Morasso
“Those treatments were developed many years ago based on the understanding at that time of certain factors involved in wound healing, but now we are beginning to tease out those factors a little bit further,” adds Dr. Morasso, the study’s senior author.
One of those factors is a molecule called FOXM1, which Dr. Morasso’s team and its collaborators at the University of Miami had previously shown to be missing-in-action in DFUs.2 That study also demonstrated that inhibiting FOXM1 in a mouse model of diabetes delays healing and decreases the abundance of certain immune cells in wounds, including cells called neutrophils that are the immune system’s ‘first responders’ to an injury. However, the mechanism through which FOXM1 influences healing remained a mystery.
In the new study, the IRP and University of Miami researchers found that inhibiting FOXM1 in neutrophils isolated from the blood of healthy volunteers caused the cells to produce more neutrophil extracellular traps (NETs), web-like meshes of various chemicals that destroy harmful invaders like viruses and bacteria. The same thing occurred in wounds in a mouse model of diabetes when the animals were given a FOXM1 inhibitor.
“The main reason that NET production occurs is to prevent the spread of infection, but when there’s excessive amounts of NETs, it can actually damage the tissue,” Dr. Sawaya explains. “What’s actually impairing wound healing is too much NET production.”
Further experiments showed that neutrophils with inactivated FOXM1 had lower activity in genes that suppress the production of reactive oxygen species (ROS), highly reactive molecules known to wreak havoc in the body. This led to higher ROS levels in the cells. Combined with the study’s other findings, this suggests that the high blood sugar levels characteristic of diabetes somehow decrease the amount of FOXM1 in neutrophils, which causes a boost in ROS levels in the cells that encourages them to produce an excessive number of NETs, resulting in impaired healing.
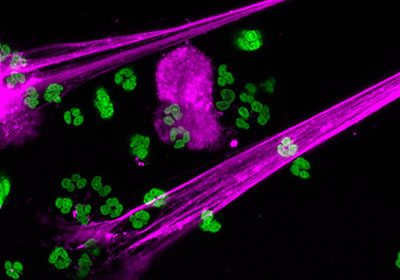
Image credit: Christian Con Yost, University of Utah
Immune cells called neutrophils produce neutrophil extracellular traps (purple) to prevent infection.
To figure out why diabetes leads to an absence of FOXM1 in neutrophils, the researchers compared the activity of various genes in the DFUs of human patients and in the skin wounds of healthy individuals. Among the numerous differences they discovered, one stood out as particularly intriguing: a gene called TREM1, which is usually highly active in neutrophils, was much less active in DFUs than in the wounds of healthy individuals. What’s more, in a mouse model of diabetes, the researchers found that treating wounds with a substance that boosts the function of TREM1 increased the number of neutrophils with high levels of FOXM1 at the site of the injury, decreased the amount of NETs those neutrophils produced, and caused the wounds to heal faster. On the other hand, increasing TREM1 activity in the wounds of non-diabetic mice had none of these effects.
Finally, in a group of people with diabetes, the researchers compared TREM1 activity and NET levels in DFUs that healed in a timely manner and those that did not. They discovered that TREM1 was less active in tissue taken from DFUs that did not heal compared to tissue from those that did, and the poorly healing wounds also contained higher amounts of neutrophils producing NETs. This suggests that TREM1 could be used as a biomarker to predict whether a specific patient’s DFU is likely to heal properly on its own or require more significant intervention.
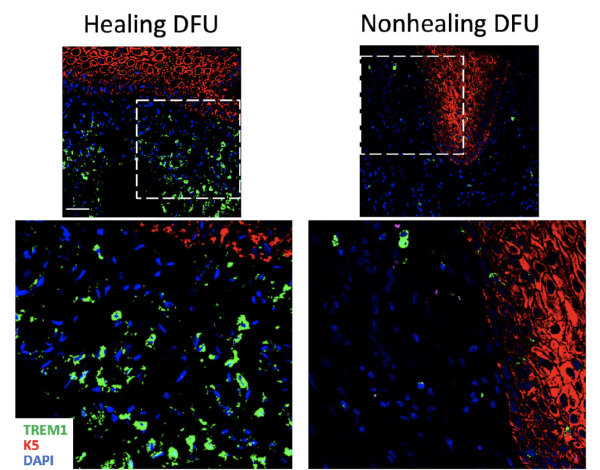
This image from the study shows higher levels of the molecule created by the TREM1 gene (green) in DFUs that healed compared to those that did not.
“Right now, it takes four or five weeks for clinicians to know whether their patients are healers or non-healers,” Dr. Morasso says. “Knowing a month ahead of time is a very big advantage for patients and clinicians.”
In addition to helping doctors predict patients’ outcomes, the study’s results suggest that treatments designed to boost FOXM1 levels or rev up the TREM1 gene could help DFUs heal. However, before such therapies can be developed, Dr. Morasso’s team and other scientists will need to learn more about how the interplay between TREM1, FOXM1, and NET production influences healing.
“We know that TREM1 is active in other types of cells that are very important not only for normal wound healing, but also in the context of diabetic non-healing wounds,” Dr. Morasso says. “The more we know about how TREM1 is doing this, the better able we’ll be to apply it for therapeutics.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
References:
[1] Sawaya AP, Stone RC, Mehdizadeh S, Pastar I, Worrell S, Balukoff NC, Kaplan MJ, Tomic-Canic M, Morasso MI. FOXM1 network in association with TREM1 suppression regulates NET formation in diabetic foot ulcers. EMBO Rep. 2022 Aug 3;23(8):e54558. doi: 10.15252/embr.202154558.
[2] Sawaya APm Stone RC, Brooks SR, Pastar I, Jozic I, Hasneen K, O’Neill K, Mehdizadeh S, Head CR, Strbo N, Morasso MI, Tomic-Canic M. Deregulated immune cell recruitment orchestrated by FOXM1 impairs human diabetic wound healing. Nat Commun. 2020 Sep 16;11(1):4678. doi: 10.1038/s41467-020-18276-0.
Related Blog Posts
This page was last updated on Wednesday, May 24, 2023
