Drug Duo Stokes Body’s Fat-Burning Furnace
Mouse Study Suggests Treatment Strategy Could Benefit Patients with Obesity

In a new IRP study, two drugs worked synergistically to rev up the ability of obese mice to burn fat and tamp down inflammation in their bodies.
It’s bad enough that the excess abdominal fat carried by most individuals with obesity causes widespread inflammation throughout their bodies. To add insult to injury, that inflammation actually makes it harder to use up overloaded fat stores. However, a new IRP mouse study supports a two-pronged pharmaceutical approach that could break this vicious cycle and help improve the health of people with obesity.1
Fat is an organ just like the liver or kidneys, and it is similarly capable of producing chemicals that cause effects throughout the body. Moreover, fat cells are not all the same; the main function of ‘white fat’ is to store excess calories in the form of molecules called triglycerides, while ‘brown fat’ uses stored triglycerides to produce heat.
“A white fat cell is an amazing cell,” says IRP investigator Aaron Cypess, M.D., Ph.D., the new study’s senior author. “It can grow up to 10 times its original size to store triglycerides, but we push this as far as we can until it bends and then breaks. If you have too much fat stored in a fat cell, the cell doesn’t like it.”

Dr. Aaron Cypess, the new study’s senior author
Over-stuffed fat cells produce molecules that trigger inflammation throughout the body, which contributes to many of the long-term health risks that plague people with obesity, including type 2 diabetes and heart disease. What’s more, this systemic inflammation disrupts the body’s ability to use stored fat for fuel. White fat cells release their stored triglycerides into the blood when chemicals called catecholamines bind to specific sites on the cells’ surface, known as beta-adrenergic receptors, and the same system spurs brown fat to burn triglycerides and generate heat. Unfortunately, inflammation causes a phenomenon called catecholamine resistance that disrupts this process. Just like a car with a busted engine won’t move very fast no matter how hard you slam on the accelerator, catecholamine-resistant fat cells expel or use fewer triglycerides when stimulated by catecholamines.
Fortunately, there are ways to stimulate even catecholamine-resistant fat cells to release stored fat. In mice, a good way to do this is with a molecule called CL-316,243 — CL for short — that stimulates beta-adrenergic receptors. In his lab’s new study, Dr. Cypess and his team examined the effects of combining CL with an anti-inflammatory dietary supplement called alpha-lipoic acid. The researchers fed mice a high-fat diet for 15 weeks and divided them into four groups: one group received CL alone for the final five weeks of the experiment, one was given only alpha-lipoic acid, one was treated with a combination of CL and alpha-lipoic acid, and one received a placebo treatment.
While the placebo group gained weight over the course of those five weeks, the mice given just CL or just alpha-lipoic acid remained essentially at the same weight, and the mice given both lost a small amount of weight, mainly from their fat stores. Since the mice in all four groups ate the same number of calories during this period, the researchers concluded that CL and alpha-lipoic acid increased the animals’ energy expenditure. CL by itself also improved the animals’ sensitivity to the blood sugar-regulating hormone insulin, which the cells of people with type 2 diabetes ignore. Alpha-lipoic acid, on the other hand, had no such effect.
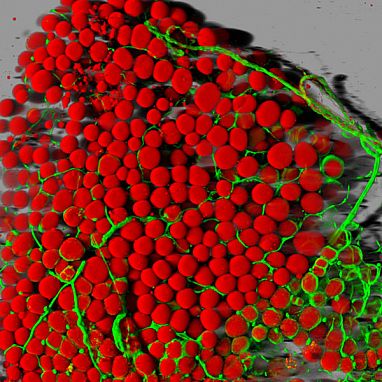
Image credit: Daniela Malide, NIH National Heart, Lung, and Blood Institute (NHLBI)
Mouse fat cells (red) and blood vessels (green).
Where the combination treatment truly shined was in its ability to reduce inflammation in the type of white fat found in mice that behaves most like the problematic abdominal fat in people with obesity. This inflammation was significantly lower in the mice that received the combination treatment compared to mice that received just CL or alpha-lipoic acid alone or the placebo treatment. The researchers theorized that this might be due to more anti-inflammatory immune cells being drawn to the fat tissue, since the fat cells of the mice given the combination treatment had higher levels of a molecule associated with that variety of immune cell.
“By themselves, alpha-lipoic acid and CL do certain things, but when you put them together, you get a significant reduction in pro-inflammatory molecules and an increase an anti-inflammatory molecules,” Dr. Cypess says. “We are still working on defining the mechanism, but part of it is probably that the alpha-lipoic acid is reducing the inflammation and making it less problematic, and that allows the CL to work better.”
In addition, compared to the other three groups, mice given the combination treatment had higher levels of a protein called UCP-1 in their white fat cells. UCP-1 is generally found at very low levels in calorie-storing white fat but high levels in calorie-burning brown fat, and the more UCP-1 a cell contains, the more energy it will burn when it is stimulated by catecholamines. Similarly, in the mice given the combination treatment, more of the molecules responsible for burning stored fat were activated via a process called phosphorylation, which would explain the animals’ increased energy expenditure.
Even if it does not cause weight loss, a treatment strategy similar to the one used in Dr. Cypess’ new study might tamp down inflammation in patients with obesity, potentially reducing their long-term risk of developing complications like heart disease and type 2 diabetes.
Because the study only used male mice, the results will need to be replicated with female animals. Nevertheless, the findings have encouraged Dr. Cypess’ team to test a similar approach on patients with obesity using alpha-lipoic acid and a drug called mirabegron that stimulates beta-adrenergic receptors in humans in the same way that CL does in mice. His lab’s prior research has already demonstrated that high doses of mirabegron increase fat burning and improve cholesterol levels and insulin sensitivity in individuals with obesity,2 but Dr. Cypess believes that adding alpha-lipoic acid to the mix could allow mirabegron to be effective at lower doses, leading to fewer side effects.
“The goal ultimately would be for people to take mirabegron at a safe dose and boost its effect with alpha-lipoic acid,” Dr. Cypess says. “If you take two safe pills and mix them together, you have a much better chance of coming up with a long-term strategy.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
References:
[1] Sater ZA, Cero C, Pierce AE, Lea HJ, Sater HA, Zhu KY, Liu N, Ma Y, Gavrilova O, Cypess AM. Combining a β3 adrenergic receptor agonist with alpha-lipoic acid reduces inflammation in male mice with diet-induced obesity. Obesity. 2022 Jan; 30(1):153-164. doi: 10.1002/oby.23309.
[2] O’Mara AE, Johnson JW, Linderman JD, Brychta R, McGehee S, Fletcher LA, Fink YA, Kapuria D, Cassimatis TM, Kelsey N, Cero C, Sater ZA, Piccinini F, Baskin AS, Leitner BP, Cai H, Milli CM, Dieckmann W, Walter M, Javitt NB, Rotman Y, Walter PJ, Ader M, Bergman RN, Herscovitch P, Chen KY, Cypess AM. Chronic mirabegron treatment increases human brown fat, HDL cholesterol, and insulin sensitivity. J Clin Invest. 2020 May 1;130(5):2209-2219. doi: 10.1172/JCI131126.
Related Blog Posts
This page was last updated on Tuesday, May 23, 2023
