Experimental Compound Supercharges Cellular Power Plants
Treatment Approach Could Combat Obesity and Its Consequences

New IRP research has identified a promising therapeutic strategy that could combat obesity and related metabolic diseases by increasing cellular energy production.
When your phone or laptop is low on power, you simply connect it to a charger and find the nearest electrical outlet, but the process of restoring lagging energy production in our cells is not nearly as simple. However, a new IRP study has identified a promising approach for doing just that, which could lead to new treatments for obesity and related metabolic ailments like heart disease and diabetes.1
Our cells rely on energy-producing mitochondria to keep them running, and cellular energy levels are constantly monitored by an enzyme called AMPK that becomes more active when energy supplies are low. To combat this low-energy state, AMPK increases mitochondrial energy production and revs up a ‘quality control’ system called mitophagy that breaks down old, defective mitochondria and creates new ones.
On the other hand, when mitochondria malfunction, they not only struggle to produce energy, but they also create reactive oxygen species (ROS) that harm cells. This is a serious concern particularly in individuals with obesity. Since AMPK only ramps up mitophagy when energy is scarce, their abundant stores of energy in the form of body fat and stockpiled glycogen, a form of sugar, depress AMPK activity.
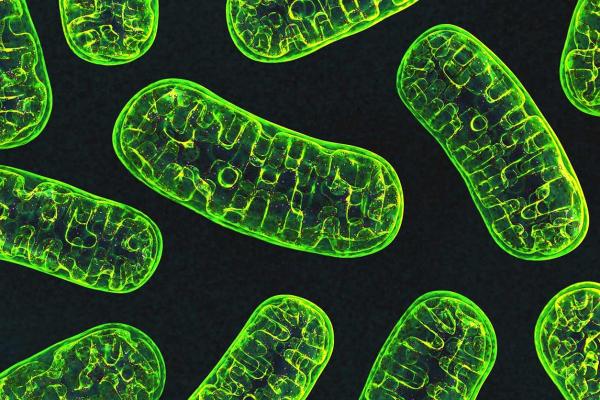
Cells rely on mitochondria (pictured here) to produce energy. These cellular energy factories are continuously recycled via a process called mitophagy.
“One of the problems that occurs with obesity is, because there’s such a surplus of energy, mitochondria production, as well as quality control, declines because you don’t need those energy factories to be working very efficiently or very hard,” explains IRP senior investigator Jay Chung, M.D., Ph.D., whose lab studies the relationship of AMPK to obesity and aging. “The cell pays less attention to mitochondria because it doesn’t need them as much, and when you neglect the quality control of mitochondria, you get an accumulation of damaged mitochondria, which can produce ROS and cause many of the diseases that stem from obesity: diabetes, heart disease, and so forth.”
Unfortunately, drugs that aim to treat those diseases by activating AMPK directly have a highly problematic side effect: they cause the heart to grow larger, which poses serious health risks and can potentially lead to heart failure. Luckily, nearly a decade ago, Dr. Chung’s team discovered an alternative way to activate AMPK.2 They found that inhibiting an enzyme called PDE4, a member of a family of proteins called phosphodiesterases (PDEs), revved up AMPK and mitophagy, resulting in improved mitochondrial function, physical stamina, and blood sugar regulation in mice, as well as protection from diet-induced obesity. What’s more, unlike other PDEs, PDE4 is not found in the heart, so drugs that target it should not cause the same heart problems as direct AMPK activators.

More From the IRP
Blog Post
Scientists Douse Fat Burning to Combat Cancer
In a new study, Dr. Chung solicited the help of a pair of organic chemists to synthesize several brand-new molecules whose structures suggested they could inhibit PDE4. Subsequent tests revealed that one of these compounds, named CBU91, only inhibited PDE4 and not its close cousins, and it was also much more effective at inhibiting PDE4 than the drug Dr. Chung’s lab used in its prior study.
“If you could find a safe AMPK activator, it would be an incredible drug, and in fact pharmaceutical companies have been working on it for decades,” Dr. Chung says. “However, it’s much more difficult to find a drug that will activate an enzyme than to develop a drug that will block it. Throwing a monkey wrench into the gears to make a machine stop is a lot easier than finding a monkey wrench that you can throw into the gears and will make the machine work better.”
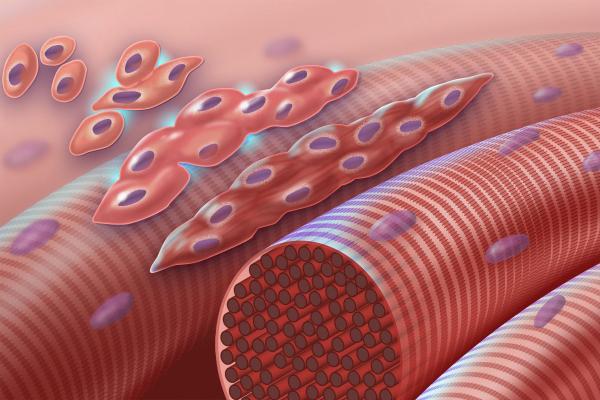
The myotubes Dr. Chung’s lab uses in its experiments closely resemble the muscle fibers, pictured here, that make up muscles in the human body.
Additional studies with collections of muscle cells called myotubes showed that CBU91 boosted AMPK activity, accelerated the breakdown of old mitochondria via mitophagy, and increased production of new mitochondria. The treated cells also showed a greater ability to burn fat molecules for fuel. Consistent with these findings, myotubes treated with CBU91 had much higher levels of messenger RNA molecules they could use to produce the PGC1-alpha protein, an enzyme that drives the production of mitochondria. Finally, because AMPK can become more active when cells die, Dr. Chung’s team ran tests that confirmed cells treated with CBU91 were not dying at a faster rate than untreated cells.
The more healthy mitochondria in cells, the greater the body’s ability to burn fat and regulate blood sugar, making drugs with effects like those of CBU91 extremely promising as treatments for obesity and diabetes. They might also be useful for combating Alzheimer’s disease because the buildup of dysfunctional mitochondria in neurons is one of the hallmarks of that devastating illness. However, because there are multiple varieties of PDE4, Dr. Chung now plans to investigate which types of PDE4 are present in different bodily tissues and develop compounds that inhibit only specific forms of it to reduce potential side effects.
“Whether you want to target the liver, or you want to target inflammatory cells, or you want to target skeletal muscle, that will determine which form of PDE4 you want to focus on,” Dr. Chung explains. “Targeting all forms of PDE4 is still too broad. We want to turn a sledgehammer into a scalpel.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
References:
[1] Potent PDE4 inhibitor activates AMPK and Sirt1 to induce mitochondrial biogenesis. Park S, Ahmad F, Bahde RJ, Philp A, Kim J, Huang T, Kim MK, Trenkle WC, Chung JH. PLoS One. 2021 June 17;16(6):e0253269. doi: 10.1177/00220345211005677.
[2] Resveratrol Ameliorates Aging-Related Metabolic Phenotypes by Inhibiting cAMP Phosphodiesterases. Park S, Ahmad F, Philp A, Baar K, Williams T, Luo H, Ke H, Rehmann H, Raussig R, Brown AL, Kim MK, Beaven MA, Burgin AB, Manganiello V, Chung JH. Cell. 2012 Feb 3;148(3):421-33. doi: 10.1016/j.cell.2012.01.017.
Related Blog Posts
This page was last updated on Wednesday, May 24, 2023
