Working Out the Chemistry of Exercise Endurance
IRP’s Paul Hwang Discovers How Muscle Cells Gear Up for Training

IRP research is revealing surprising insights into how exercise transforms our muscles.
As the weather warms up in March, which is National Athletic Training Month, many of us come out of hibernation and finally fulfill that new year’s resolution to start exercising. Deep down in our cells, our mitochondria, the tiny power stations that turn oxygen into energy, start getting a workout, too.
IRP senior investigator Paul Hwang, M.D., Ph.D., studies how mitochondria and cellular energy production affect human health and disease, with a particular focus on cardiovascular health and cancer. However, March’s many new fitness enthusiasts might be most interested in a recent finding from his laboratory that seems to explain how muscles build endurance as we train them through exercise. His team’s insights also explain why muscles revert back to couch potato mode so quickly when we stop regular exercise.
“We know a lot about the physiologic changes that occur with exercise and training, but many of the specific molecules that relay signals are less clear,” Dr. Hwang says. “Our research identified one specific part of the whole process at the molecular level.”
It's obvious to anyone who exercises regularly that routine activity changes our skeletal muscles — the ones attached to our frames that allow us to move. Scientists have long known that these changes include a boost in the amount of oxygen that muscle cells’ mitochondria can use to make adenosine triphosphate (ATP), the molecule that serves the energy needs of cells in all living organisms.
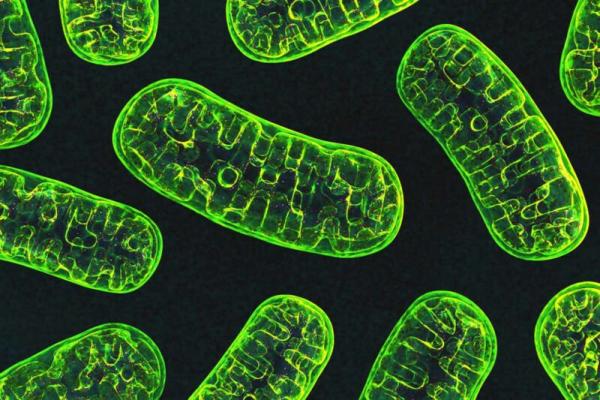
Our cells use tiny batteries called mitochondria, pictured here, to make energy.
“This finding correlates well with the observation that when you exercise, you become more ‘oxidatively fit,’ meaning your ability to use oxygen is increased,” Dr. Hwang explains. “Most of the oxygen we breathe in is used by the mitochondria to generate ATP, which is necessary to exercise our muscles. The oxygen is the crude oil, and the ATP is the gasoline.”
Dr. Hwang’s lab added to that long-standing knowledge with a discovery about a specific molecule that works in an unexpected manner to induce this change. That protein, known as CHCHD4, is integral to both building mitochondria and to regulating how those mitochondria use oxygen.
“We observed that CHCHD4 gets down-regulated during exercise, which is surprising,” Dr. Hwang says. “I say surprising because CHCHD4 is a well-known molecule critical for the formation of new mitochondria, so we didn’t expect to see a key molecule involved in building mitochondria get down-regulated,” since that means the protein is doing less in our cells, not more.
The specific types of muscle fibers that make up someone’s skeletal muscles have a big influence on that person’s physical endurance.
More specifically, Dr. Hwang’s lab found that exercise sets off a chain reaction that reduces the amount of CHCHD4 in muscle cells, which causes the long fibers that make up skeletal muscles to convert from a ‘glycolytic’ form that doesn’t use much oxygen for making ATP into to an ‘oxidative’ form that does. Because using oxygen to make ATP produces much more of it than doing so through other processes, the newly transformed muscle fibers become more resistant to fatigue.1
“Although CHCHD4 is known to help bring proteins into mitochondria, genetically reducing its dose in mouse models did not result in less mitochondria, indicating that its normal levels are excessive,” Dr. Hwang explains. “Interestingly, our study revealed another function of CHCHD4, whereby its downregulation by exercise activates a specific signaling pathway that promotes oxidative fiber formation. In the end, you actually end up with more effective mitochondria in skeletal muscle. It’s an adaptive mechanism and it’s very dynamic. It speaks to the capacity for exercise that we gain — or that we lose if we stop exercising.”
While understanding what happens at the molecular level when we exercise is interesting, Dr. Hwang’s research may also have an important clinical application: preventing or reducing obesity. In published epidemiological studies, people with more oxidative fibers in their muscles have less fat mass. In addition, scientists in Dr. Hwang’s lab and other groups have found that mice with only one functioning copy of the CHCHD4 gene don’t gain as much fat as they age as mice with two working copies, and they retain more muscle as well. Dr. Hwang’s findings explain the biological mechanism behind that relationship. Dr. Hwang hopes that it will one day be possible to make a drug to reduce CHCHD4 production or otherwise rev up the pathway through which lower CHCHD4 levels transform muscle fibers into more-efficient oxygen users, which could help people more easily maintain a healthy body weight.

Dr. Paul Hwang
In the meantime, Dr. Hwang and his research team continue to unpack the relationship between mitochondria and diseases like cancer. In fact, their discovery that a tumor suppressor gene, p53, can regulate mitochondrial function by interacting with CHCHD4 was what initially led them down the path toward learning the role CHCHD4 plays in building exercise endurance. Dr. Hwang’s lab is also looking at a gene, WASF3, that disrupts the function of mitochondria, making muscle cells unable to accommodate exercise in people with chronic fatigue syndrome.2
This ability to pursue new leads when they come up in the course of scientific research makes NIH special, according to Dr. Hwang. In many labs outside NIH, his team would not have been able to devote resources to studying a protein like CHCHD4 that they had not originally intended to investigate.
“It’s really hard to plan for discovery; instead, we follow the science, or, in this case, we follow the molecules,” Dr. Hwang says. “That’s one of the great things about NIH: we’re given the freedom to follow the science, and with my clinical background, do translational studies that bring clinical relevance to our findings about human biology.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
References:
- Ma J, Wang PY, Zhuang J, Son AY, Karius AK, Syed AM, Nishi M, Wu Z, Mori MP, Kim YC, Hwang PM. CHCHD4-TRIAP1 regulation of innate immune signaling mediates skeletal muscle adaptation to exercise. Cell Rep. 2023;43(1):113626.
- Wang PY, Ma J, Kim YC, Son AY, Syed AM, Liu C, Mori MP, Huffstutler RD, Stolinski JL, Talagala SL, Kang JG, Walitt BT, Nath A, Hwang PM. WASF3 disrupts mitochondrial respiration and may mediate exercise intolerance in myalgic encephalomyelitis/chronic fatigue syndrome. Proc Natl Acad Sci U S A. 2023;120(34):e2302738120.
Related Blog Posts
This page was last updated on Monday, March 17, 2025
