A Promising Path to Saving Stiffening Livers
IRP Study Could Lead to New Treatments for an Increasingly Prevalent Liver Ailment
IRP research has identified an important driver of liver damage that occurs when high amounts of fat build up in the organ.
Experienced bakers know certain ingredients are impossible to work with when they’re not supple enough — just try making cookies with ice-cold butter or rock-hard brown sugar. The same could be said of the liver, which struggles to do its job when high levels of fat accumulate in it and trigger a process that binds the liver up in an inflexible mesh. Recent IRP research has identified a key set of biochemical events responsible for that ailment, pointing the way towards a possible method of treating an increasingly prevalent cause of liver disease.1
It's normal and healthy for the liver to store some fat, but when it hordes too much fat — often due to a poor diet or high alcohol consumption — that can serve as an early warning for future health problems. Some people with fatty liver disease unrelated to alcohol consumption go on to develop a condition called metabolic-associated steatohepatitis (MASH), in which inflammation and cellular damage begin to occur in the liver.
“When that happens, the liver compensates with increased production of collagen, which fills in the holes in the liver where liver cells have died,” explains IRP senior investigator Frank Gonzalez, Ph.D., the new study’s senior author. “The body doesn’t like to have holes in the liver, so the collagen fills up that space and causes the liver to become very stiff, and that really affects the function of the liver.”
In a small proportion of MASH patients, the condition can continue to worsen until the liver develops serious scarring and even liver cancer, so the swelling ranks of people with MASH portend a future with much higher rates of severe liver disease. In order to avoid that unfortunate scenario, doctors will need a way to prevent or reverse the excessive production of collagen in the liver, known as fibrosis.
“Companies have been trying for years to develop drugs to treat MASH,” Dr. Gonzalez says. Although a new, first-of-its-kind drug to treat MASH was recently approved by the U.S. Food and Drug Administration, it has some significant limitations and doesn’t work for all patients, so more effective drugs with fewer side effects are still needed.
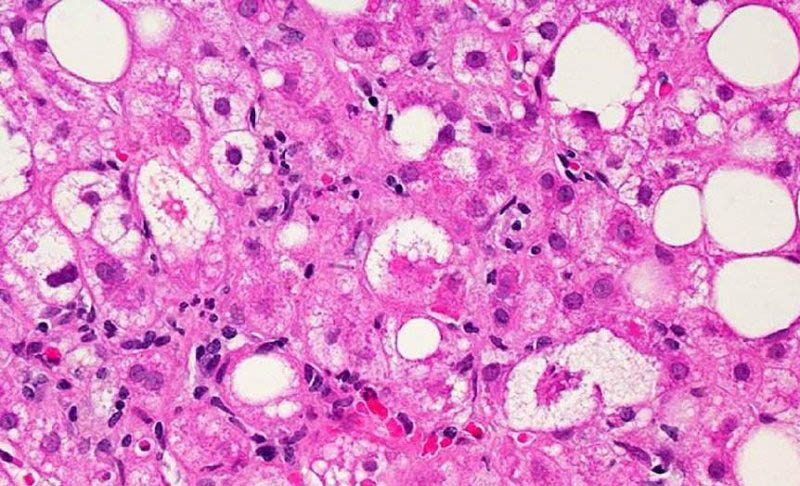
A microscopic image of liver tissue affected by MASH. The large and small white spots are excess fat droplets filling liver cells. Photo credit: Dr. David Kleiner, NIH National Cancer Institute
The new study from Dr. Gonzalez’s lab points to a promising new avenue for that research. The work kicked off when the study’s first author, Tingting Yan, Ph.D., noticed something surprising while working as a visiting postdoctoral fellow from China in Dr. Gonzalez’s lab. Dr. Yan observed that inactivating a gene called CEBPA in the liver cells of adult mice with MASH caused an increase in liver fibrosis that looked eerily similar to the way MASH worsens in humans. The CEBPA gene codes for a protein of the same name that regulates the activity of many other genes.

Dr. Frank Gonzalez (left) and Dr. Tingting Yan (right)
“There was a very marked enhancement of liver fibrosis in CEBPA knockout mice,” Dr. Yan recalls. “The livers looked very bad — so different from the livers of matched, genetically normal mice — but their body weight didn’t change, which means this gene knockout didn’t affect obesity, but it really enhanced the stiffness of the liver. That convinced me that this gene must have a huge effect on liver fibrosis.”
To follow up on that discovery, a team of researchers led by Drs. Yan and Gonzalez probed the links between the CEBPA gene and liver fibrosis. They found that when the gene was less active in human liver tissue, other genes involved in fibrosis and inflammation were more active. In liver tissue from MASH patients, the CEBPA gene was less active than in liver tissue from healthy individuals, and patients with more severe MASH had lower levels of the CEBPA protein in their livers. Similarly, levels of the CEBPA protein dropped in the livers of mice fed a diet designed to induce MASH.

As highly processed foods spread around the world, increasing rates of obesity are likely to spur a corresponding increase in MASH cases, making research into treatments even more important.
The scientists followed up on those findings by revisiting the mice that first spurred Dr. Yan’s interest in the CEBPA gene, which could be given a chemical to inactivate the gene in their livers as adults. Once again, doing that caused the mice to develop significant liver fibrosis, which was made even worse when the mice were fed a MASH-promoting diet.
Further experiments revealed that a lack of the CEBPA protein altered the activity of numerous genes. The gene that revved up the most with less CEBPA around was the Spp1 gene, which provides instructions for making a protein called osteopontin. When the researchers deleted the CEBPA gene in isolated cells called hepatocytes taken from the livers of mice, the Spp1 gene became massively more active in the cells and levels of osteopontin increased. On the other hand, increasing the activity of the CEBPA gene in those cells led to less Spp1 activity and osteopontin production.
“When CEBPA is lost in hepatocytes during MASH development, the Spp1 gene is also very impressively upregulated,” Dr. Yan says. “Hepatocyte-derived osteopontin is a very powerful driver of liver fibrosis.”
Finally, the researchers tested the effects of a gene therapy designed to introduce extra copies of the CEBPA gene into the liver. They fed mice with intact CEBPA genes a MASH-inducing diet for six months and treated the mice with the gene therapy halfway through that period. Within just three weeks of the treatment, CEBPA levels increased in the animals’ livers, while the Spp1 gene had slowed its production of osteopontin. By the end of the study period, the treated mice also had noticeably lower levels of fat accumulation and fibrosis in their livers compared to untreated mice.

The IRP-led study suggests that giving liver cells extra copies of the CEBPA gene may be one way to prevent or reverse liver fibrosis.
“When we repopulate the animals’ livers with CEBPA, we can resolve MASH,” Dr. Gonzalez says.
With these discoveries in hand, researchers can begin pursuing treatments for MASH that either boost CEBPA levels in patients’ livers or directly suppress the production of osteopontin by certain liver cells. In addition, the same strategy might turn out to be useful for treating liver fibrosis caused by either high levels of alcohol consumption or a viral infection, although further research is needed to investigate that possibility. Still more therapeutic strategies may be discovered by figuring out why large fat stores in the liver suppress the activity of the CEBPA gene, a mystery Dr. Gonzalez’s lab is keen to solve.
“If you could find that pathway and then block it, that would be another way to treat MASH,” he says. “Fats accumulate in the liver over the course of time, based on diet and obesity and other factors, and these fats somehow are causing a decrease in certain proteins in the liver, including CEBPA, and that leads to the disease. That’s kind of the black box that we’re in: what regulates the regulator?”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
References:
[1] Yan T, Yan N, Xia Y, Sawaswong V, Zhu X, Dias HB, Aibara D, Takahashi S, Hamada K, Saito Y, Li G, Liu H, Yan H, Velenosi TJ, Krausz KW, Huang J, Kimura S, Rotman Y, Qu A, Hao H, Gonzalez FJ. Hepatocyte-specific CCAAT/enhancer binding protein α restricts liver fibrosis progression. J Clin Invest. 2024 Apr 1;134(7):e166731. doi: 10.1172/JCI166731.
Related Blog Posts
This page was last updated on Tuesday, June 11, 2024
