Scientific Detour Advances Understanding of Fatty Liver Disease
IRP Study Suggests a Way to Prevent Unhealthy Fat Buildup
IRP scientists have discovered that mice lacking an enzyme called Smurf1 stored much more fat in their livers. The results point towards a potential treatment approach for non-alcoholic fatty liver disease (NAFLD) in humans.
Many important scientific discoveries happen when a scientist stumbles across something curious and decides to investigate further. Alexander Fleming, for example, famously discovered penicillin by examining mold that grew in one of his petri dishes while he was away on vacation. A recent IRP study spurred by a similarly unexpected observation could eventually lead to a method of preventing or reversing unhealthy amounts of fat storage in the liver.1
Despite the many businesses hawking ‘fat-blasting’ diets and workouts, storing fat in the body is actually necessary for good health. However, the way fat affects the body depends on its quantity and location. Excessive fat storage in the liver, known as fatty liver disease, can eventually lead to serious health problems like impaired liver function and, in rare cases, liver cancer.
Fatty liver disease can be caused by consuming too much alcohol, but tens of millions of Americans have non-alcoholic fatty liver disease (NAFLD), which is linked to unhealthy diets and obesity. Doctors typically recommend weight loss to their NAFLD patients because there are no medications currently approved for the condition. However, that may one day change because of new research led by IRP senior investigator Ying Zhang, Ph.D.
Dr. Zhang’s laboratory studies a particular pathway thought to play a role in cancer and many other diseases. Roughly a decade ago, her group created genetically engineered mice that lacked the gene for a key molecule in that system, called Smurf1. Eliminating that gene in one particular breed of mouse, they noticed, caused large amounts of fat to build up in the liver.
“That was something we were not expecting at all,” Dr. Zhang says. “We were looking for effects on this other pathway, not for this liver condition, but it kept showing up.”
Over time, the mice without Smurf1 — but not mice lacking a related protein called Smurf2 — accumulated much more fat in their livers than normal mice, though they retained normal liver function. They also had other traits commonly found in human NAFLD patients, including a higher body weight, more body fat, and problems regulating their blood sugar.
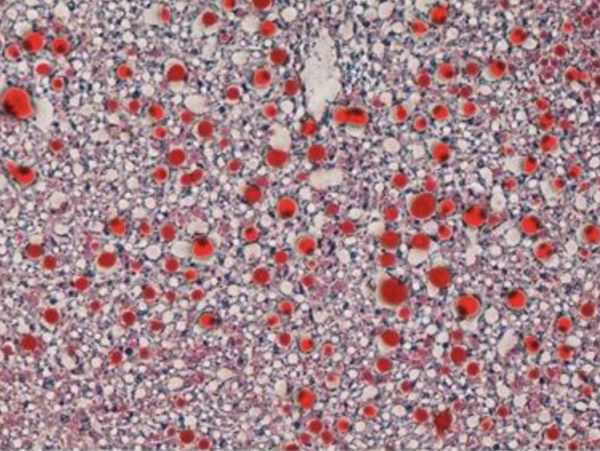
A chemical stain of liver tissue from mice without Smurf1 shows large fat deposits (red).
A subsequent analysis of gene activity in the animals’ livers found nearly a thousand genes that were substantially more or less active in the mice without Smurf1 than in normal mice. Among them was the gene for a molecule called PPAR-gamma, which drives the activity of several other genes important for regulating fat in the body. PPAR-gamma also boosts the activity of its own gene, so higher levels of PPAR-gamma promote even greater production of PPAR-gamma. In liver cells from normal mice, knocking down levels of Smurf1 increased levels of PPAR-gamma and boosted the activity of those fat-regulating genes. The researchers ultimately discovered that this occurs because Smurf1 can attach a certain chemical tag to the PPAR-gamma molecule, and this stops PPAR-gamma from revving up those fat-controlling genes. Without Smurf1, PPAR-gamma is free to drive the activity of those genes to higher and higher levels.
Finally, the researchers gave a compound that inhibits PPAR-gamma to mice without Smurf1 before they had accumulated large amounts of liver fat. The treatment normalized the amount of fat in the animals’ livers, along with decreasing their body weights and body fat to an amount similar to normal mice. The treatment also restored typical activity in several fat-regulating genes that are influenced by PPAR-gamma.
“That was the final step to really show that increased PPAR-gamma activity is not just an unrelated consequence of Smurf1 loss but actually the cause of this fatty liver condition,” Dr. Zhang says.
Dr. Zhang’s team also found that lower activity in the gene that produces Smurf1 was associated with a higher body mass index in humans. This suggests that Smurf1 might affect fat regulation in humans as well. Her research team plans to continue working with mice lacking Smurf1 to better gauge their similarities to human NAFLD patients, with an eye towards using the findings to help develop therapies for the condition.
“The intramural funding, research facilities, and collegial working environment of NIH offer a superb place for pursuing unexpected scientific leads like the one that led to this study,” Dr. Zhang says. “I am fortunate to be able to take the ‘free-wheeling’ support of the IRP to pursue my interest in fatty liver disease that resulted from a serendipitous observation.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
References:
[1] Non-proteolytic ubiquitin modification of PPARγ by Smurf1 protects the liver from steatosis. Zhu K, Tang Y, Xu X, Dang H, Tang LY, Wang X, Wang XW, Zhang YE. PLoS Biol. 2018 Dec 19;16(12):e3000091. doi: 10.1371/journal.pbio.3000091.
Related Blog Posts
This page was last updated on Tuesday, January 30, 2024
