A New Approach to Male Birth Control
Research in Cells Shows Promise for an Alternative Way to Halt Sperm Production
Men may be one step closer to having a birth control pill of their own thanks to experiments in cells recently conducted by IRP researchers.
Birth control has long been mostly one-sided, as the vast majority of contraceptive methods are intended exclusively for women. However, recent IRP research has shown the potential of a new approach towards creating a reversible method of male contraception.1
Women have a vast array of contraceptive options available to them, from ‘the pill’ to intrauterine devices (IUDs) and other products. However, for men, the only options aside from condoms are safe but irreversible surgical procedures. More than 40 percent of pregnancies in the U.S. are unintended, and additional options for male birth control could help reduce that number.
More than two decades ago, the lab of IRP senior investigator Maria Dufau, M.D., Ph.D. — the new study’s senior author — discovered a multi-functional protein called gonadotropin-regulated testicular RNA helicase (GRTH/DDX25) that is present at high levels in the testes of mice, rats, and humans.2,3 Nearly ten years later, her team identified a genetic mutation in a small group of infertile men that affects the gene responsible for producing GRTH.4 Rather than shutting down the production of GRTH entirely, the mutation instead prevents GRTH from undergoing a process called phosphorylation, in which a molecule called PKA attaches a chemical tag to a certain part of GRTH. Dr. Dufau’s team also found that mice with a particular mutation in the GRTH gene completely lacked phosphorylated GRTH and could not produce mature sperm, making them infertile.5
“Without GRTH phosphorylation, we do not get sperm,” Dr. Dufau explains. “This approach to male contraception was natural for us because we found that GRTH is a convenient protein for studying and is also essential for the production of mature sperm.”
In the new study, Dr. Dufau’s lab teamed up with IRP computational physicist Sergio A. Hassan, Ph.D., who helped them leverage computer modeling to design compounds that would block the site where PKA phosphorylates GRTH. The researchers opted to synthesize and test molecules called cyclic peptides in which the components form a circular chain, as opposed to a linear one. They went with this approach not only because cyclic peptides tend to be less toxic than other compounds, but also because of the particular structure of the site where PKA phosphorylates GRTH.
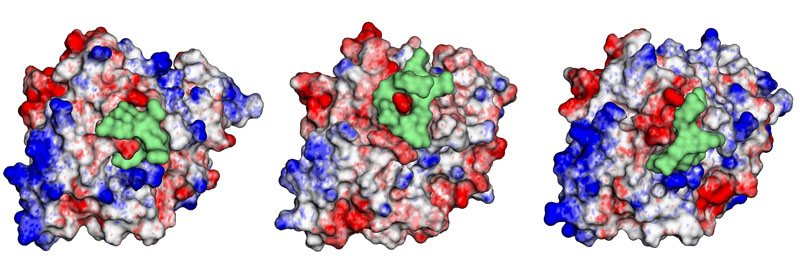
These images from Dr. Hassan’s computer modeling show the researchers’ cyclic peptides (green) binding to GRTH (red, white, and blue).
“What we found is that there is not a real pocket there,” Dr. Dufau says. “It’s more like a flat surface, so you cannot use a drug that fits perfectly inside a pocket. You have to use a cyclic peptide that is more accommodating to that surface.”
Her team’s experiments confirmed that their cyclic peptides were non-toxic and capable of entering mammalian cells. Moreover, in cells manipulated to over-produce phosphorylated GRTH, cyclic peptides designed to block GRTH phosphorylation succeeded in doing so, whereas peptides that did not target the site of GRTH phosphorylation had no effect. The same results were seen in isolated seminiferous tubules, the tiny tubes in the testicles where sperm are created.
Seminiferous tubules in the testes of mice
In another experiment, Dr. Dufau’s team revved up cells’ production of PKA in order to boost their levels of phosphorylated GRTH. However, two of the cyclic peptides the group designed blunted that increase in phosphorylated GRTH, though levels were still higher than in cells with less PKA. This supported the researchers’ theory that their cyclic peptides were reducing GRTH phosphorylation by preventing GRTH from interacting with PKA.
“The cyclic peptides don’t inhibit the activity of PKA,” Dr. Dufau explains. “Rather, they compete with PKA for binding to the surface of GRTH.”
Though the study only included experiments in isolated cells, when combined with Dr. Dufau’s past research, the findings suggest that cyclic peptides that inhibit GRTH phosphorylation could one day be used to stop men from producing sperm. For now, Dr. Dufau’s team plans to examine how its cyclic peptides affect sperm counts, fertility, and behavior in mice, as well as whether the compounds’ effects reverse themselves when the treatment is stopped. Creating a ‘male birth control pill’ also presents numerous obstacles that will have to be overcome, such as creating a compound to put in the pills that does not get destroyed by the stomach and can cross the blood-testis barrier.
“This is like the blood-brain barrier, but it’s even better,” Dr. Dufau says. “It’s a perfect barrier. We have to figure out whether this compound will go through the barrier. If not, we’ll have to engineer some way to get it through.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
References:
[1] Blockade of GRTH/DDX25 Phosphorylation by Cyclic Peptides Provides an Avenue for Developing a Nonhormonal Male Contraceptive. Raju M, Kavarthapu R, Anbazhagan R, Hassan SA, Dufau ML. J Med Chem. 2021 Oct 14;64(19):14715-14727. doi: 10.1021/acs.jmedchem.1c01201.
[2] Gonadotropin Regulation Testicular RNA Helicase, Two Decades of Studies on Its Structure Function and Regulation From Its Discovery Opens a Window for Development of a Non-hormonal Oral Male Contraceptive. Dufau ML, Karvarthapu R. Front Endocrinol. 2019 Aug 29;10:576. doi: 10.3389/fendo.2019.00576.
[3] A novel gonadotropin-regulated testicular RNA helicase. A new member of the dead-box family. Tang PZ, Tsai-Morris CH, Dufau ML. J Biol Chem. 1999 Dec 31;274(53):37932-40. doi: 10.1074/jbc.274.53.37932.
[4] Polymorphism of the GRTH/DDX25 gene in normal and infertile Japanese men: a missense mutation associated with loss of GRTH phosphorylation. Tsai-Morris CH, Koh E, Sheng Y, Maeda Y, Gutti R, Namiki M, Dufau ML. Mol Hum Reprod. 2007 Dec;13(12):887-92. doi: 10.1093/molehr/gam065.
[5] Targeted knock-in mice with a human mutation in GRTH/DDX25 reveals the essential role of phosphorylated GRTH in spermatid development during spermatogenesis. Kavarthapu R, Anbazhagan R, Raju M, Tsai-Morris CH, Pickel J, Dufau ML. Hum Mol Genet. 2019 Aug 1;28(15):2561-2572. doi: 10.1093/hmg/ddz079.
Related Blog Posts
This page was last updated on Wednesday, May 24, 2023
