Scientists Douse Fat Burning to Combat Cancer
Inhibiting Energy Production Pathway Delays Tumor Formation in Mice

Both healthy and cancerous cells normally stoke their furnaces by using a variety of substances for fuel. Decreasing their ability to burn fat molecules for energy delayed the appearance of tumors in cancer-prone mice, new IRP research found.
Despite the common misconception that sugary treats send kids bouncing off the walls, fat actually provides more than twice as much energy as sugar and other carbohydrates. This energy can be a double-edged sword, fueling not just healthy cells but also cancerous ones. A new IRP study in mice suggests that reducing the body’s ability to burn fat molecules for energy could slow the formation of tumors, potentially extending the lives of individuals with strong genetic predispositions to cancer.1
One of the most commonly mutated genes in cancer cells is the TP53 gene, which produces a protein called p53 that assists the repair of damaged DNA and prevents runaway cellular division. In some rare cases, individuals are born with a TP53 mutation that affects all of their cells, leading to a condition called Li-Fraumeni syndrome (LFS) that dramatically increases their risk of developing numerous types of cancer early in life. Consequently, doctors who treat people with LFS screen them frequently for cancer so tumors can be dealt with as soon as they appear.
“The TP53 gene is probably one of the most highly studied genes, yet there’s no treatment that prevents or delays cancer in Li-Fraumeni syndrome,” says IRP senior investigator Paul Hwang. M.D., Ph.D., the new study’s senior author.
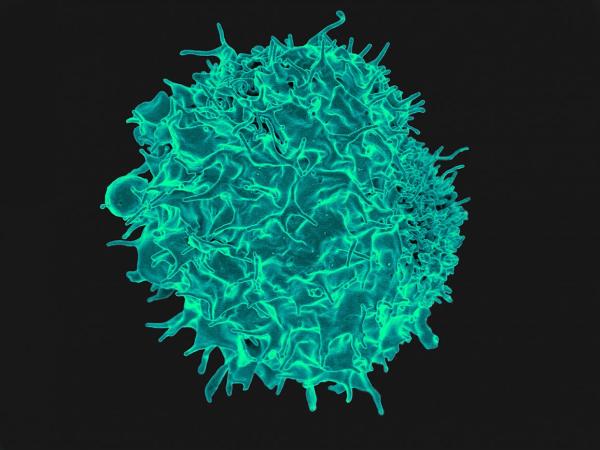
A T cell, the type of immune cell that tends to turn cancerous in mice with genetic mutations similar to those found in patients with Li-Fraumeni Syndrome.
In 2013, Dr. Hwang’s research group published a study that examined people with LFS and mice with mutations in their Trp53 genes, the mouse equivalent of the human TP53 gene. These mice have a tendency to develop lymphoma, a cancer affecting immune cells called T cells, early in their lives. The study found that both the mice and the human patients had more active mitochondria, the cellular components that produce energy.2 A subsequent study, published in 2017, found that tamping down mitochondrial activity in those mice caused them to develop cancer much later in life than they normally would.3
In the new study, Dr. Hwang’s team focused in particular on the process by which mitochondria produce energy from fat molecules, known as fatty acid oxidation. In a set of mice with mutated Trp53 genes, the scientists also knocked out the gene for a protein called myoglobin that helps transport oxygen in cells, an important step for fatty acid oxidation (hence the ‘ox’ in ‘oxidation’). Compared to mice with normal Trp53 genes, mice with mutated Trp53 genes had elevated fatty acid oxidation in their cancer-prone T cells. However, mice with mutated Trp53 and myoglobin genes had lower levels of fatty acid oxidation than animals that only had a Trp53 mutation. Moreover, eliminating the myoglobin gene in mice with a Trp53 mutation delayed the development of lymphoma, leading to them living more than 40 percent longer on average, though most of the mice still eventually developed lethal lymphoma.
More From the IRP
Blog Post
Synthetic Antibody Rallies Immune Cells Against Ovarian Cancer
Further experiments revealed that mice with mutated Trp53 genes had more activity in a signaling pathway that is important for cell growth and which tumors often use to fuel their expansion. However, eliminating the myoglobin gene put the brakes on this revved-up growth system, providing a possible explanation for why decreased fatty acid oxidation might delay cancer development.
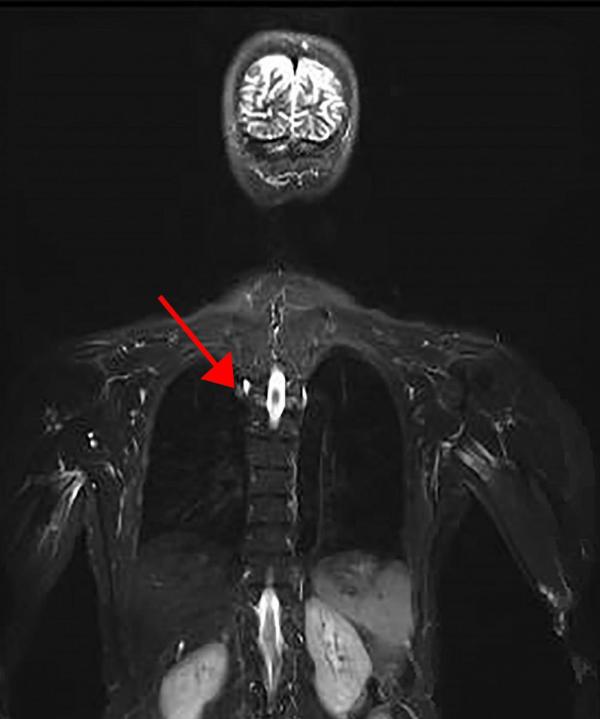
Representative image from a whole-body magnetic resonance imaging (MRI) scan of a patient with LFS. The arrow points to a cancerous tumor in the lungs.
The scientists also selectively knocked out one copy of a gene called CPT2 in the T cells of a separate set of mice with mutated Trp53 genes. CPT2 is critical for fatty acid oxidation, and mice with a Trp53 mutation whose T cells also had only one working copy of the CPT2 gene had reduced fatty acid oxidation in those cells and lived roughly 30 percent longer than mice with mutated Trp53 genes and the normal set of two intact CPT2 genes.
“The myoglobin is like a hammer — you’re affecting the whole body globally and changing metabolism in all the different systems,” explains Dr. Hwang. “However, we demonstrated that if you just selectively downregulate fatty acid oxidation in the cells that become cancerous — in this case, the T cells — then you affect tumor formation.”
The study’s results could contribute to changes in how doctors care for patients with LFS, as well as other groups with a high risk for certain cancers. Medications already exist that generally tamp down mitochondrial activity, such as the diabetes drug metformin, and so do drugs that inhibit fatty acid oxidation in particular, though they tend to have significant side effects. Researchers at the National Cancer Institute (NCI) plan to evaluate whether metformin might help delay the development of cancer in LFS patients, and additional studies will be needed to determine if using a drug to specifically reduce fatty acid oxidation provides benefits that outweigh the potential side effects.
“When you mildly inhibit mitochondrial function or fatty acid oxidation, you initiate a sort of cell braking system,” Dr. Hwang says. “If we can use that approach to delay cancer, that would be a great thing, especially because we could use drugs that are already available.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
References:
[1] Reducing fatty acid oxidation improves cancer-free survival in a mouse model of Li-Fraumeni syndrome. Wang P, Ma J, Li J, Starost MF, Wolfgang MJ, Singh K, Pirooznia M, Kang J, Hwang PM. Cancer Prev Res (Phila). 2020 Sep 21. doi: 10.1158/1940-6207.CAPR-20-0368.
[2] Increased oxidative metabolism in the Li-Fraumeni syndrome. Wang P, Ma W, Park J, Celi FS, Arena R, Choi JW, Ali QA, Tripodi DJ, Zhuang J, Lago CU, Strong LC, Talagala SL, Balaban RS, Kang J, Hwang PM. N Engl J Med. 2013 Mar 14;368(11):1027-32. doi: 10.1056/NEJMoa1214091.
[3] Inhibiting mitochondrial respiration prevents cancer in a mouse model of Li-Fraumeni syndrome. J Clin Invest. 2017 Jan 3;127(1):132-136. doi: 10.1172/JCI88668.
Related Blog Posts
This page was last updated on Tuesday, May 23, 2023
