Inflammation Cuts Lifeline for Blood-Producing Stem Cells
Discovery Could Lead to New Approaches for Boosting Blood Cell Counts
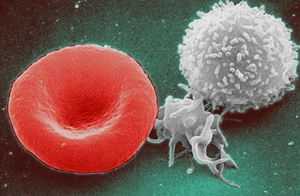
An adequate supply of blood cells like red blood cells (left), platelets (middle), and white blood cells (right) is critical for good health. New IRP research has discovered how an inflammatory molecule reduces the production of these cells and why a particular drug relieves the problem.
Much of human biology is a black box — scientists know the key players and the end results, but not how those outcomes come about. Consequently, it remains a mystery why some medications help patients. A new IRP study has cracked open the black box to reveal how high levels of an inflammatory molecule inhibit blood cell production in some individuals and why a particular medicine helps reverse this life-threatening condition.1
The study was spurred by a series of clinical trials for patients with aplastic anemia, a condition that results from the death of cells in the bone marrow called hematopoietic stem and progenitor cells (HSPCs). These cells eventually turn into the various types of cells found in the blood: oxygen-carrying red blood cells, disease-fighting white blood cells, and blood-clotting platelets. In patients with aplastic anemia and similar disorders, high levels of an inflammatory immune molecule called interferon gamma cause HSPCs to die off. This happens because interferon gamma inhibits a process triggered by a hormone called thrombopoietin (TPO) that keeps HSPCs alive.
Several NIH clinical trials conducted over the past decade showed that a TPO-mimicking drug called eltrombopag increases levels of all three varieties of blood cells in patients with aplastic anemia, a surprising finding given that eltrombopag was originally developed only to correct low levels of platelets but not red or white blood cells.2,3,4 The findings suggested that eltrombopag was helping the HSPCs survive and generate all three blood cell types, just like TPO does. NIH investigator Andre Larochelle, M.D., Ph.D., found it surprising that a TPO substitute was helping aplastic anemia patients, who generally have even more TPO than healthy people.5
“It was remarkable that, despite high levels of TPO, these patients responded to eltrombopag, which mimics TPO,” says Dr. Larochelle, the new study’s senior author. “We were essentially giving more TPO to patients who already had too much TPO.”
The first set of experiments in Dr. Larochelle’s new study showed, as expected, that many more HSPCs died when they were exposed to a combination of interferon gamma and TPO compared to when eltrombopag replaced the TPO. Next, the scientists showed that interferon gamma prevented TPO from triggering a cellular process that keeps HSPCs alive, but it did not stop eltrombopag from initiating that same chain of events. Dr. Larochelle theorized that interferon gamma was increasing the activity of proteins that act as a brake on that process, but he ultimately found that those proteins behaved the same way regardless of whether TPO or eltrombopag activated them.
“These findings were unexpected and led us to seek an alternative explanation for the negative impact of interferon gamma on HSPCs,” Dr. Larochelle says.
The researchers ultimately discovered that interferon gamma binds to TPO and prevents it from interacting with the cellular receptor on HSPCs that it needs to in order to keep the cells alive. However, interferon gamma cannot similarly bind to eltrombopag, leaving the drug free to interact with the TPO receptor even when TPO cannot. This finding resolved the mystery of why TPO-mimicking eltrombopag helps aplastic anemia patients who already have high levels of TPO in addition to high levels of interferon gamma.
While eltrombopag was already a standard treatment for aplastic anemia, the new findings suggest that the medication could help patients with similar conditions caused by high levels of inflammatory molecules like interferon gamma. Dr. Larochelle has already begun enrolling patients in a clinical trial to test if eltrombopag helps patients with a rare genetic disease called Fanconi anemia.
“Our findings bring a completely new way to think about how inflammation impacts cells in general,” Dr. Larochelle says. “Inflammation is a common condition that can lead to many disorders, and the concept uncovered in our study could potentially be applied for the treatment of other diseases caused by chronic inflammation.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
References:
[1] Eltrombopag maintains human hematopoietic stem and progenitor cells under inflammatory conditions mediated by IFNγ. Alvarado LJ, Huntsman HD, Cheng H, Townsley DM, Winkler T, Feng X, Dunbar CE, Young NS, Larochelle A. Blood. 2019 Feb 25. pii: blood-2018-11-884486. doi: 10.1182/blood-2018-11-884486.
[2] Eltrombopag and improved hematopoiesis in refractory aplastic anemia. Olnes MJ, Scheinberg P, Calvo KR, Desmond R, Tang Y, Dumitriu B, Parikh AR, Soto S, Biancotto A, Feng X, Lozier J, Wu CO, Young NS, Dunbar CE. N Engl J Med. 2012 Jul 5;367(1):11-9. doi: 10.1056/NEJMoa1200931.
[3] Eltrombopag restores trilineage hematopoiesis in refractory severe aplastic anemia that can be sustained on discontinuation of drug. Desmond R, Townsley DM, Dumitriu B, Olnes MJ, Scheinberg P, Bevans M, Parikh AR, Broder K, Calvo KR, Wu CO, Young NS, Dunbar CE. Blood. 2014;123(12):1818-1825.
[4] Eltrombopag Added to Standard Immunosuppression for Aplastic Anemia. Townsley DM, Scheinberg P, Winkler T, Desmond R, Dumitriu B, Rios O, Weinstein B, Valdez J, Lotter J, Feng X, Desierto M, Leuva H, Bevans M, Wu C, Larochelle A, Calvo KR, Dunbar CE, Young NS. N Engl J Med. 2017 Apr 20;376(16):1540-1550. doi: 10.1056/NEJMoa1613878.
[5] Cytokine signature profiles in acquired aplastic anemia and myelodysplastic syndromes. Feng X, Scheinberg P, Wu CO, Samsel L, Nunez O, Prince C, Ganetzky RD, McCoy JP Jr, Maciejewski JP, Young NS. Haematologica. 2011 Apr;96(4):602-6. doi: 10.3324/haematol.2010.030536. Epub 2010 Dec 15.
Related Blog Posts
This page was last updated on Tuesday, January 30, 2024
