Crucial Protein Reins in Overzealous Bone Growth
IRP Study Answers Key Questions About Bone Development and Healing

A recent IRP study sheds light on the role of a key molecule responsible for ensuring bones bounce back strong after a fracture.
The idea that there can be “too much of a good thing” applies just as much to the human body as it does to an overly sweet dessert or excessive holiday decorations. For instance, you might think that rapid bone growth would be helpful for fixing fractures, but it can actually make bones weaker in the long run. A recent IRP study revealed how a certain molecule manages the way bones develop in a growing fetus and heal after damage to make sure they don’t trade strength for speed.1
Rather than being lone wolves, our bones depend on a more elastic type of tissue, called cartilage, that caps their ends and cushions them during movement. In fact, when new bone forms in utero or during healing, it starts life as cartilage before being converted into hardened bone. What’s more, both bone and cartilage secrete a variety of substances that form a critical support structure known as the ‘extracellular matrix.’ Bones without the extracellular matrix are like a rainforest without insects — the jungle may look lush at first glance, but the lack of tiny critters can eventually cause big problems.
Out of all the proteins found in bones’ extracellular matrix, IRP senior investigator Marian Young, Ph.D., has a particular fondness for one called biglycan. After all, she was one of several IRP scientists who first discovered it in the early 1980s.
“We discovered other proteins besides biglycan, but that’s the one we focused on because it was unique — no one was studying it,” she explains. “We were sort of trailblazing.”

Dr. Young (right) examines a plastic replica of the human skull with a postbaccalaureate fellow in her lab.
In addition to discovering biglycan and determining that it is abundant in bones and teeth, Dr. Young’s lab also created a type of mouse in the 1990s that lacked the gene for biglycan. These mice had lower bone mass than their genetically normal counterparts, but until recent it was unclear why.
In her lab’s new study, Dr. Young and her IRP team made huge strides toward answering that question. They began by examining how bones form when mouse embryos are developing in the womb. They discovered that mouse embryos without the gene for biglycan had fewer cells called chondrocytes, which are critical for creating cartilage. They also had bone-producing cells called osteoblasts that were less mature, which compromised their ability to build strong bone. These developmental differences set the mice up for significant problems later in life, as six-week-old biglycan-deficient mice had bones that were softer and had abnormal structures, including fewer and thinner girder-like rods called trabecula. These findings help explain why people with rare mutations in the biglycan gene have weakened bones, and they could also help lead to new treatments for other conditions that have similar consequences.
“You always want to know, when someone has a mutation that affects their bones, where did things go wrong,” Dr. Young says. “There is a human bilgycan mutation and these patients do have low bone mass, and our findings hint that might occur very early in development.”
At six weeks of age, the bones of mice without biglycan (right) had structural abnormalities that made them weaker than the bones of genetically normal mice (left).
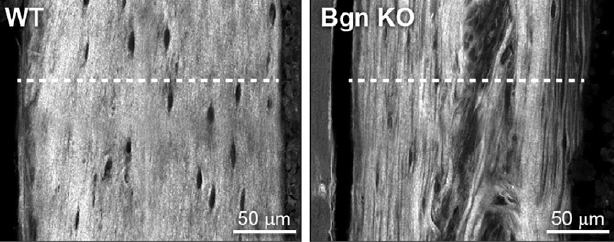
Of course, we need to build strong bones not only during the earliest stages of life, but also after accidents cause our bones to break. The healing process is kick-started when immune cells are drawn to the site of a bone fracture. The resulting inflammation causes the thin layer of stem cells, blood vessels, and nerves surrounding bones, known as the ‘periosteum,’ to grow rapidly. However, Dr. Young’s new study showed that in adult mice without biglycan, fewer of those immune cells showed up at the site of a fracture, so the periosteum did not grow as much as it normally does. The fractures in those mice also generated noticeably less cartilage while healing than in mice with biglycan, instead quickly and prematurely producing bone. Just like in the experiments with mouse embryos, this resulted in weaker bones with abnormal structures and less supportive elements in their extracellular matrix.
“The cells without biglycan are somehow skipping that cartilage step and making bone too early, and when we look at its character, it’s not normal,” Dr. Young says. “It’s like when you go to the grocery store and buy a kit to make a cake rather than making it from scratch yourself. You can make the cake faster, but it’s often not as good.”

As part of the new study, the IRP scientists grew bone-producing stem cells with and without biglycan on three-dimensional scaffolds like this one.
What’s more, when Dr. Young’s team grew bone-producing stem cells from the periosteum on a three-dimensional scaffold, the scientists observed that stem cells without the gene for biglycan did not develop into cartilage-producing chondrocyte cells as often as genetically normal stem cells, leading to lower amounts of cartilage in the resulting three-dimensional structures. When those structures were subsequently implanted under the skin of healthy, genetically normal mice, the structures without biglycan ended up with more bone and less cartilage than the ones with biglycan, mirroring what the scientists observed during bone healing in biglycan-deficient mice.
Ultimately, the results of the study could help not only people with brittle bones caused by certain genetic mutations, but also people with more common bone ailments. For example, when people lose teeth because they have lost bone mass in their jaws, dentists sometimes graft bone from elsewhere in the body onto the jaw to strengthen it and may use a substance naturally produced in the body, called bone morphogenic protein (BMP), to accelerate the growth of the new bone in the jaw. Dr. Young speculates that adding biglycan to BMP might help the healing process even more after such procedures and make the resulting bone more resilient.
Of course, more research in cell and animal models will be needed before biglycan-based treatments can be tested in people. However, none of those studies will be led by Dr. Young, who retired at the end of May. After more than three decades spent studying bone development at NIH and “putting biglycan on the map,” she says, she is looking forward to seeing how other scientists build on her work.
“You put your research out there and anyone can read it, and people jump on and take it where they want to take it,” she says. “I’m happy to confer with them — as long as I’m not at the beach.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
References:
[1] Shainer R, Kram V, Kilts TM, Doyle AD, Shainer I, Martin D, Simon CG Jr, Zeng-Brouwers J, Schaefer L, Young MF, Genomics and Computational Biology Core. Biglycan regulates bone development and regeneration. Front Physiol. 2023 Feb 16;14:1119368. doi: 10.3389/fphys.2023.1119368.
Related Blog Posts
This page was last updated on Monday, August 21, 2023
