Ultrasound-Based Technique Produces Fertile Ground for Therapeutic Stem Cells
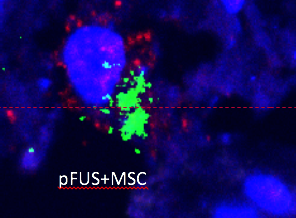
This close-up image of kidney tissue shows therapeutic stem cells called mesenchymal stromal cells (MSCs), whose energy-producing mitochondria are shown in red. The MSCs are releasing a chemical that aids healing, shown in green. New IRP research has revealed why a medical technology called pulsed focused ultrasound (pFUS) boosts the healing powers of these stem cells.
Every good gardener knows the importance of fertilizing the soil before planting seeds, and evidence is accumulating that a similar concept applies to the human body when it comes to experimental stem cell therapies. A new IRP study has uncovered how a medical technology called pulsed-focused ultrasound boosts the healing potency of a particular stem cell treatment.1
Stem cells are a focus of intense scientific interest because their ability to morph into a variety of cell types makes them useful for repairing damaged tissue. In addition, some types of stem cells, like the mesenchymal stromal cells (MSCs) that come from bone marrow, might also be beneficial because they secrete chemicals that influence the immune system’s response to disease and injury.
While MSC infusions on their own have been shown to enhance recovery from numerous ailments, including kidney injury and tissue damage from reduced blood flow to the limbs, the lab of IRP senior investigator Joseph A. Frank, M.D., the new study’s senior author, discovered that MSC treatment is even more effective when paired with pulsed focused ultrasound (pFUS)2,3. A variant of the ultrasound imaging technology used in fetal sonograms to examine developing babies, pFUS hits tissues with high-intensity sound waves for therapeutic applications. Past studies by Dr. Frank’s team have revealed that pFUS acts like both a net and a fertilizer, increasing the number of MSCs that gather in the area of the body exposed to focused ultrasound and improving the infused cells’ therapeutic effects when they get there4,5.
“The literature concerning the effectiveness of MSC therapy is wide ranging: some studies suggest it works like a charm, some say it doesn’t do anything, and numerous other results are everywhere in between,” says Scott Burks, Ph.D., a staff scientist in Dr. Frank’s lab and the new study’s first author. “The aim our group is pursuing is that pulsed-focused ultrasound might make the difference between an MSC therapy being minimally effective or robustly effective.”
In their new study, Dr. Frank and Dr. Burks set out to determine why pFUS makes MSC treatment more effective. They focused on the roles of two chemicals: one called interferon-gamma that tissues produce more of after exposure to pFUS6 and another called interleukin-10 that MSCs release to tamp down the immune response and promote healing. The researchers began by demonstrating that MSCs isolated from donated human bone marrow and grown in their lab secreted more interleukin-10 when exposed to interferon-gamma. When injected into mice with damaged kidneys, those interferon-gamma-treated MSCs released more interleukin-10 when they reached the injured organs and spurred greater healing compared to MSCs not exposed to interferon-gamma beforehand.
The researchers then applied pFUS or fake, ‘sham’ ultrasound to the kidneys of mice with kidney damage prior to giving them MSC injections. Some of the mice were genetically altered ‘knockouts’ that could not produce interferon-gamma, and some of the genetically normal mice were given MSCs that could not make interleukin-10.
pFUS increased the number of MSCs that gathered in the kidneys of both typical and knockout mice, and both sets of mice benefitted from the MSC treatment even when they did not receive pFUS. Moreover, these effects occurred regardless of whether or not the infused MSCs could produce interleukin-10. However, whereas the combination of pFUS and MSC injections was more therapeutically beneficial than MSCs alone in the genetically normal mice, receiving pFUS did not enhance the therapeutic potency of MSC treatment in the mice that could not make interferon-gamma. Similarly, genetically normal mice given interleukin-10-deficient MSCs also did not get any extra clinical benefit from the addition of pFUS.
All together, these results demonstrate that exposing damaged kidneys to pFUS boosts the therapeutic effects of MSC injections for two reasons: pFUS causes more MSCs to collect in the kidneys and spurs the kidneys to produce more interferon-gamma, which causes the MSCs that amass there to release more interleukin-10.
“In essence, you’re not only increasing the number of therapeutic units at the site, but also making them more therapeutically active,” says Dr. Burks.
The research team plans to further refine their innovative combination treatment by investigating whether certain subtypes of MSCs provide more clinical benefit than others, as well as honing in on the best ways to apply pFUS to make MSC injections more effective. Eventually, they hope their approach could be used to treat a wide variety of conditions, including brain and heart injuries.
“This study is a proof-of-principle from a technical standpoint,” Dr. Burks says, “but we envision this approach being broadly applicable to nearly any condition for which you would choose MSCs for therapy.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
References:
[1] Mesenchymal stromal cell potency to treat acute kidney injury increased by ultrasound-activated interferon-γ/interleukin-10 axis. Burks SR, Nagle ME, Bresler MN, Kim SJ, Star RA, Frank JA. J Cell Mol Med. 2018 Sep 14. doi: 10.1111/jcmm.13874.
[2] Improving the therapeutic efficacy of mesenchymal stromal cells to restore perfusion in critical limb ischemia through pulsed focused ultrasound. Tebebi PA, Kim SJ, Williams RA, Milo B, Frenkel V, Burks SR, Frank JA. Sci Rep. 2017 Feb;7:41550. doi: 10.1038/srep41550.
[3] Pulsed focused ultrasound pretreatment improves mesenchymal stromal cell efficacy in preventing and rescuing established acute kidney injury in mice. Burks SR, Nguyen BA, Tebebi PA, Kim SJ, Bresler MN, Ziadloo A, Street JM, Yuen PS, Star RA, Frank JA. Stem Cells. 2015 Apr;33(4):1241-53. doi: 10.1002/stem.1965.
[4] Enhanced Homing Permeability and Retention of Bone Marrow Stromal Cells by Noninvasive Pulsed Focused Ultrasound. Ziadloo A, Burks SR, Gold EM, Lewis BK, Chaudhry A, Merino MJ, Frenkel V, Frank JA. Stem Cells. 2012 Jun;30(6):1216-1227. doi: 10.1002/stem.1099.
[5] Noninvasive Pulsed Focused Ultrasound Allows Spatiotemporal Control of Targeted Homing for Multiple Stem Cell Types in Murine Skeletal Muscle and the Magnitude of Cell Homing Can Be Increased Through Repeated Applications. Burks SR, Ziadloo A, Kim SJ, Nguyen BA, Frank JA. Stem Cells. 2013 Nov;31(11):2551-60. doi: 10.1002/stem.1495.
[6] Investigation of Cellular and Molecular Responses to Pulsed Focused Ultrasound in a Mouse Model. Burks SR, Ziadloo A, Hancock HA, Chaudhry A, Dean DD, Lewis BK, Frenkel V, Frank JA. PLoS One. 2011;6(9). doi: 10.1371/journal.pone.0024730.
Related Blog Posts
This page was last updated on Wednesday, May 24, 2023
