Modified Stem Cells Boost Regeneration After Stroke
New Technique Overcomes Major Obstacle to Stem-Cell-Based Treatments
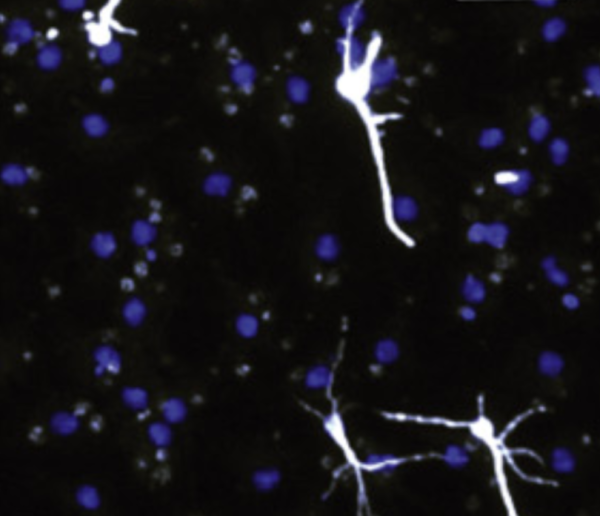
Boosting a cellular process called SUMOylation helped neural stem cells survive hostile conditions similar to those in the post-stroke brain and encouraged them to develop into neurons (white).
Your brain cells need plenty of oxygen and nutrients to survive — that is, unless you’re a hibernating ground squirrel. By tapping into the cellular process that keeps these animals’ brains healthy during the long winter months, IRP scientists have discovereda way to increase the survival of neuron-producing stem cells implanted into the brain after a stroke, a development that could one day dramatically improve stroke treatment.1
An ischemic stroke cuts off blood flow to part of the brain, depriving the cells there of life-sustaining substances. While clinicians can reduce the damage by restoring blood flow as quickly as possible, there is not yet a way to help patients regenerate lost neurons. One promising approach is to implant neural stem cells into the damaged area, which would then develop into new neurons and establish connections with nearby cells to restore function. Unfortunately, like an oil spill that makes a patch of ocean uninhabitable, a stroke creates conditions that make it difficult for stem cells to survive.
In an attempt to overcome this obstacle, the lab of IRP senior investigator John Hallenbeck, M.D., has spent more than a decade studying a process called SUMOylation that the group discovered helps brain cells survive the lack of blood flow that occurs when a certain species of squirrel hibernates.2SUMOylation alters the activity and cellular location of proteins by attaching chemical tags called small ubiquitin-like modifiers (SUMOs) to them. In theory, revving up SUMOylation in stem cells could help them survive in the hostile environment of a post-stroke brain.
“Stroke therapy is a field ripe for innovation,” says Dr. Joshua Bernstock, who led the new study as part of his graduate research in Dr. Hallenbeck’s lab. “Stem cells are really dynamic molecular medicines that can react in real-time to the conditions in the focal diseased environment, so they’re kind of like living drugs.”
In the new study, Dr. Bernstock and his colleagues utilized transgenic mice that had more of the enzyme that attaches SUMO tags to other proteins. They found that neural stem cells from the brains of these mice had higher levels of SUMOylation and less activity in genes involved in cell death. Moreover, when placed in a petri dish, the cells consumed less oxygen than neural stem cells from normal mice and more frequently developed into neurons rather than other types of brain cells.
Next, the scientists placed the transgenic stem cells and neurons that grew from them into a petri dish that lacked oxygen and nutrients. After five hours, the cells were re-exposed to those life-sustaining substances. Compared to stem cells and neurons with typical levels of SUMOylation, significantly more of the transgenic stem cells and neurons survived this experiment, which approximates the conditions in the brain caused by an ischemic stroke.
Finally, the research team examined the survival and behavior of neural stem cells implanted into the brains of mouse models of ischemic stroke. Once again, more of the transgenic cells survived the hostile conditions of the post-stroke brain. In addition, those cells produced more neurons than did normal stem cells, and those neurons formed more connections with neighboring cells.
“The same phenomenon that we had seen in the petri dish played out in the brains of the mice,” Dr. Bernstock explains. “That’s very promising when you think about how to engineer a cell that you would ultimately hope to use in the context of regenerative medicine.”
Future studies will need to examine how grafts of neural stem cells with greater SUMOylation affect functional recovery in mouse models of stroke. Scientists might also look into methods of boosting SUMOylation that don’t involve manipulating genes. Ultimately, if the approach is proven to be safe and effective in animals, clinicians may one day implant neural stem cells with increased SUMOylation into the brains of stroke patients to help them regenerate lost neurons.
“As a physician-scientist, it’s always exciting to be working on an experimental therapeutic that has clinical implications,” Dr. Bernstock says. “As we continue to refine these techniques and define the mechanisms by which stem cells help protect and repair the brain, I’m incredibly optimistic that these sorts of preclinical studies will advance from the bench to the bedside.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
References:
[1] SUMOylation promotes survival and integration of neural stem cell grafts in ischemic stroke. Bernstock JD, Peruzzotti-Jametti L, Leonardi T, Vicario N, Ye D, Lee YJ, Maric D, Johnson KR, Mou Y, Van Den Bosch A, Winterbone M, Friedman GK, Franklin RJM, Hallenbeck JM, Pluchino S. EBioMedicine. 2019 Mar 21. pii: S2352-3964(19)30175-6. doi: 10.1016/j.ebiom.2019.03.035. [Epub ahead of print]
[2] Protein SUMOylation is massively increased in hibernation torpor and is critical for the cytoprotection provided by ischemic preconditioning and hypothermia in SHSY5Y cells. Lee Y, Miyake S, Wakita H, McMullen DC, Azuma Y, Auh S, Hallenbeck JM. J Cereb Blood Flow Metab. 2007 May;27(5):950-962. doi: 10.1038/sj.jcbfm.9600395.
Related Blog Posts
This page was last updated on Tuesday, January 30, 2024
