Research Reveals Trigger for Brain’s Repairmen
IRP Discoveries Could Enhance Recovery from Brain Injuries

New IRP research has revealed the trigger for specific cells in the brain to repair blood vessels damaged by a stroke, traumatic brain injury, or infection.
Many futurists and science fiction writers dream about a time when nanobots will run around our bodies fixing any damage that occurs. Until that day comes, we’re reliant on our immune system to mop up when things go wrong, a fickle set of cells that sometimes needs a push to get going. IRP scientists recently discovered how a particular type of cell in the blood stimulates the brain’s construction crew to leap into action, potentially opening the door to treatments that boost healing in the brain.1
The brain is greedy, hoarding 20 percent of the body’s oxygen even though it only makes up 2 percent of the body’s mass. An intricate matrix of blood vessels supplies the brain with all that oxygen, but when an insult like a traumatic brain injury, an infection, or a stroke occurs, the brain’s blood vessels can rupture. This ‘cerebrovascular injury,’ or CVI, not only compromises oxygen delivery to the brains’ neurons, but blood leaking from damaged vessels can also directly kill those cells.
“With all these types of injuries, the faster you get that damage resolved, the better off you will be,” says IRP senior investigator Dorian McGavern, Ph.D., the new study’s senior author. “Having blood vessels continue to leak for days or weeks or months is really bad for your brain.”
Scientists have long known that immune cells that live in the brain, called microglia, play a crucial role in recovery from a CVI. However, Dr. McGavern’s IRP team recently added to this understanding with the discovery of a specific population of microglia in mice that helps rebuild damaged blood vessels. Dr. McGavern’s team dubbed these cells ‘repair-associated microglia,’ or RAMs for short, and found that they are distinct from other microglia because they produce high amounts of a substance called vascular endothelial growth factor A (VEGFA).2
“That’s the quintessential factor needed to help rebuild blood vessels, and it gets produced basically every time you’re building new blood vessels,” Dr. McGavern explains. “We found that the sole source of VEGFA was microglia, so that’s what locked us into these cells as being the critical players in rebuilding damaged blood vessels.”
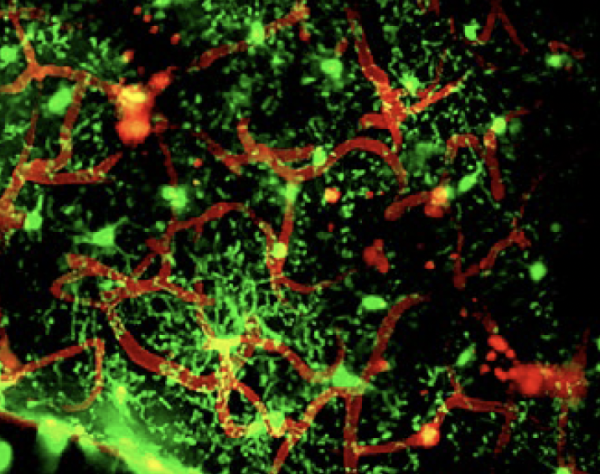
In a mouse model of CVI, microglia (green) swarmed around damaged blood vessels (red) to help seal them up.
Despite that important insight, it remained a mystery what causes run-of-the-mill microglia to throw on their toolbelts and become RAMs. In his lab’s new study, Dr. McGavern’s team first confirmed that RAMs exist in the human brain by identifying microglia that produce lots of VEGFA in human brain tissue taken from the site of a stroke. Then, they used a mouse model of CVI — the same one in which they first discovered RAMs — to dig into the sequence of events that follows such an injury. They found that lots of RAMs clustered at the location of a CVI within six days of the injury, and the more severe the injury, the more RAMs assembled there. What’s more, those cells not only produced lots of VEGFA, but also had a receptor on their surfaces for an important immune system chemical called interleukin 6 (IL-6).
RAMs were not the only immune cells that responded to the CVI, though. Dr. McGavern’s team also found that within three days of a CVI, immune cells called monocytes traveled from the blood to the brain, where they secreted IL-6 in order to transform typical microglia into RAMs. When those monocytes were unable to produce IL-6 because they were missing the gene for it, the RAMs’ response to the brain injury was dramatically reduced, leading to more neuronal cell death at the site of the CVI and poorer performance on a test measuring the animals’ motor and cognitive function.

Dr. Dorian McGavern
Because monocytes are called to the brain by the activation of a specific receptor on their surface, CCR2, Dr. McGavern’s team next examined what would happen when mice without the CCR2 gene experienced a CVI. These mice had 60 percent fewer RAMs and 80 percent fewer monocytes at the site of the CVI compared to genetically normal mice. However, treating these mice with IL-6 boosted the RAMs’ response back to typical levels, curbing the loss of neurons and improving recovery of motor and cognitive function after the injury. The scientists were also able to boost RAM levels in mice without the CCR2 gene by transfusing them with monocytes from mice with intact CCR2 genes, but only when those monocytes were capable of secreting IL-6.
“There’s a couple different types of cells that can produce IL-6, but pound for pound, the number one cell in terms of producing IL-6 was the monocytes,” Dr. McGavern says. “When you don’t have the monocytes coming into the brain, the amount of IL-6 drops considerably, and when that happens the RAM programming is not sufficient.”

New treatments based on Dr. McGavern’s findings could help reduce the burden of physical and cognitive disability caused by strokes, traumatic brain injuries, and other types of CVI.
With these new discoveries in hand, scientists like Dr. McGavern can now begin developing ways to boost healing after a CVI, such as by triggering the CCR2 receptor to recruit more monocytes to the brain or using IL-6 to turn more microglia into RAMs. Dr. McGavern’s team is also exploring ways to identify which patients might require those therapies and which are likely to recover well the old-fashioned way.
“Some patients might have problems with their circulating monocytes — maybe some patients don’t have as many of them or their cells don’t produce as much IL-6 and we need to do something to help along the reparative microglia in the brain,” he says. “Those are the patients you really want to help — those who are experiencing faulty repair and are not recovering well. You want to see if you can go in and add something to that system to help it do better.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
References:
[1] Choi BR, Johnson KR, Maric D, McGavern DB. Monocyte-derived IL-6 programs microglia to rebuild damaged brain vasculature. Nat Immunol. 2023 May 29. doi: 10.1038/s41590-023-01521-1.
[2] Mastorakos P, Mihelson N, Luby M, Burks SR, Johnson K, Hsia AW, Witko J, Frank JA, Latour L, McGavern DB. Temporally distinct myeloid cell responses mediate damage and repair after cerebrovascular injury. Nat Neurosci. 2021 Feb;24(2):245-258. doi: 10.1038/s41593-020-00773-6.
Related Blog Posts
This page was last updated on Tuesday, August 15, 2023
