COVID–19 Timeline at NIH (March-April 2023)
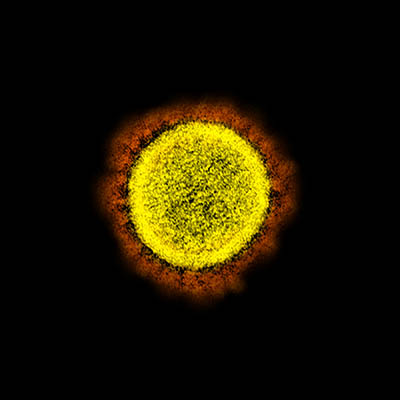
CREDIT: NIAID
Transmission electron micrograph of SARS-CoV-2 virus particles, isolated from a patient. Image captured and color-enhanced at the NIAID Integrated Research Facility in Fort Detrick, Maryland.
March 1: FBI Director Christopher Wray states his agency’s stance that the COVID-19 pandemic may have resulted from an accidental laboratory leak in China.
March 1: A study by NIA scientists found that a protein crucial to the life cycle of SARS-CoV-2 has a new role in the virus’ replication and genetic proofreading process (J Biol Chem 299:102980, 2023).
March 3: The CDC updates its COVID-19 community levels: Rocky Mountain Laboratories in Hamilton, Montana, moves from medium to high community level; Phoenix, Arizona, moves from medium to low; Framingham, Massachusetts, moves from low to medium; all other NIH locations remain at their current levels.
March 8: A House Select Subcommittee begins hearings investigating the origins of the coronavirus pandemic.
March 6: The Building 12B cafeteria and Building 50 coffee bar resume service at NIH’s Bethesda, Maryland, campus.
March 10: The CDC updates its COVID-19 community levels: Rocky Mountain Laboratories in Hamilton, Montana, moves from high to medium community level; Framingham, Massachusetts, moves from medium to low; all other NIH locations remain at their current levels.
March 10: The CDC announces that it will no longer require travelers from China to present a negative COVID-19 test before boarding their flights to the United States.
March 11: Former NIAID Director Anthony Fauci speaks to CNN to discuss the minority view that the COVID-19 virus occurred as a result of work in a Chinese lab.
March 14: The NIH Clinical Center announces that the plexiglass shields in the north and south lobbies have been removed and that the round circles in the elevators (also known as Twister decals) may also be removed soon.
March 14: The FDA expands the emergency use authorization of the Pfizer BioNTech SE’s bivalent COVID-19 vaccine as a single booster dose in children six months through four years of age who have completed their initial three-dose vaccination with Pfizer’s original shot.
March 17: The CDC updates its COVID-19 community levels: Rocky Mountain Laboratories in Hamilton, Montana, moves from medium to high community level; all other NIH locations remain the same.
March 20: A NIAID-supported study finds that prior SARS-CoV-2 infection weakens immune-cell response to vaccination and suggests the need to boost CD8+ T cell response after infection (Immunity 2023; DOI:10.1016/j.immuni.2023.03.005).
March 24: The CDC updates its COVID-19 community levels: Rocky Mountain Laboratories in Hamilton, Montana, moves from high to medium community level; all other NIH locations remain at their current levels.
March 29: A study by NIDA and other collaborators found that the expanded availability of opioid use disorder-related telehealth services and medications during the COVID-19 pandemic was associated with a lowered likelihood of fatal drug overdose among Medicare beneficiaries (JAMA Psychiat 2023; DOI:10.1001/jamapsychiatry.2023.0310).
March 31: The CDC updates its COVID-19 community levels: Rocky Mountain Laboratories in Hamilton, Montana, moves from medium to low community level; all other NIH locations remain the same.
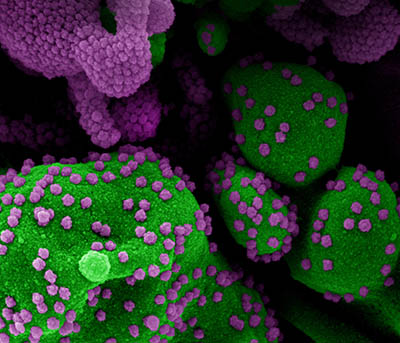
CREDIT: NIAID
Colorized scanning electron micrograph of an apoptotic cell (green) heavily infected with SARS-CoV-2 virus particles (purple), isolated from a patient sample. Image from the NIAID Integrated Research Facility in Fort Detrick, Maryland.
April 10: The Clinical Center updates its COVID guidance. Asymptomatic patients undergoing any procedures, including high-risk aerosol-generating procedures, are no longer required to undergo SARS-CoV-2 preprocedure testing. All patients will continue to be screened for symptoms and recent exposure to SAR-CoV-2 using the emerging infection screening tool before any procedures.
April 10: President Joseph Biden signs a bipartisan congressional resolution to end the COVID-19 national emergency, bringing it to a close after three years and weeks before it was set to expire alongside a separate public health emergency. On January 31, 2020, then-Health and Human Services Secretary Alex Azar first declared a public health emergency and then-President Donald Trump declared the COVID-19 pandemic a national emergency that March.
April 10: The Biden administration launches Project Next Gen, a $5 billion-plus program to accelerate development of new coronavirus vaccines and treatments to better protect against a still-mutating virus and to protect against other coronaviruses that might be a future threat.
April 18: The FDA releases updated guidance to simplify the use of COVID-19 vaccines: Adults who have not yet been vaccinated will get one dose of an updated bivalent shot; seniors and immunocompromised individuals may get another booster of the bivalent vaccine at least four months following their first dose of the bivalent vaccine; children 6 months through 5 years of age may receive a bivalent vaccine, but the number of doses that they receive will depend on the vaccine and their vaccination history.
April 24: The NIH Clinical Center returns to in-person quarterly Town Halls in the Lipsett Amphitheater. Three sessions will be held at 7:30 a.m., 1:00 p.m., and 5:30 p.m. The 1:00 p.m. session will be videocast for those unable to attend in person and archived for later viewing.
April 25: An NIH-funded study finds that children with multisystem inflammatory syndrome, a rare condition linked with the virus that causes COVID-19, have biochemical indicators of cell injury and cell death that are distinct from other children with COVID-19 (Cell Rep Med 2023; DOI:10.1016/j.xcrm.2023.101034).
This page was last updated on Thursday, May 4, 2023
